Abstract
Purpose
To evaluate the feasibility of ultrasound in detecting spontaneous pneumomediastinum in the neonatal intensive care unit (NICU) and illustrate the ultrasound features.
Methods
Among neonates with abnormal mediastinal radiolucency suspected on chest radiography, those referred for ultrasound examination within 2 days were included. Anterior mediastinal ultrasound was performed using a linear transducer (5–12 MHz) to determine the presence and location of abnormal air in the mediastinum. Clinical data for the neonates were also reviewed.
Results
On ultrasound, pneumomediastinum appeared as thick linear/curvilinear echogenic lines, some with posterior shadowing located between the anterior chest wall and thymus, in lateral margins of the thymus, between the thymus and the great vessels, and in the middle of the thymic parenchyma.
Conclusions
Using ultrasound, pneumomediastinum was easily visualized, and localization of the abnormal air accumulation was possible. Ultrasound may be used as a radiation-free supplementary imaging modality for neonates with abnormal mediastinal air.
Similar content being viewed by others
Introduction
Neonatal pneumomediastinum occurs in approximately 2.5 per 1,000 live births [1]. The underlying mechanism is alveolar rupture with leakage of air into the lung interstitium and dissection of that gas into the hilum and subsequently the mediastinum [2]. Most spontaneous pneumomediastinum in neonates is associated with birth injury, prematurity, pneumonia, meconium aspiration syndrome or assisted ventilation [3]. The infant is often asymptomatic or presents with mild respiratory distress, and the condition usually resolves spontaneously [4]. The diagnosis of pneumomediastinum is usually based on chest radiography and physical examination. However, chest radiography may not be diagnostic, especially when there is only a small amount of gas collection in the thoracic cavity. In such cases, ultrasound may be helpful in demonstrating the presence of a small amount of abnormal air in the mediastinum because the relatively unossified thoracic cavity of neonates along with the presence of a large thymus allows a good acoustic window [5, 6]. Despite many advantages of ultrasound in neonates, there is little information regarding the use of ultrasound for evaluation of abnormal air in the mediastinum. The purpose of this study is to investigate the feasibility of using ultrasound to detect neonatal spontaneous pneumomediastinum and to describe the sonographic features.
Materials and methods
The study included consecutive neonates hospitalized in the NICU between April 2010 and November 2010. Neonates with abnormal mediastinal radiolucency on chest radiography were identified. Among these neonates, those referred for ultrasound study of the brain or abdomen within 2 days of radiolucency detected on chest radiography were enrolled. Unstable neonates and neonates with extensive pneumomediastinum limiting evaluation of the air distribution on ultrasound were excluded. Approval was given by our Institutional Review Board.
Mediastinal sonograms were obtained after performing routine scanning of the referred brain or abdomen area. Mediastinal sonography was performed with the neonates in the supine position using a 5- to 12-MHz linear array transducer (iU22, Philips Medical Systems, Bothell, WA, USA). The transducer was placed directly over the anterior chest wall to visualize the thymus and great vessels. During the sonographic examination, several transverse and longitudinal scans were obtained to assess the presence and distribution of the abnormal air. The hospital records of the neonates were reviewed for age at ultrasound examination, sex, gestation at delivery, birth weight, mean Apgar score, mode of delivery, presence of underlying conditions such as meconium aspiration, respiratory symptoms, and clinical course.
Results
Among 870 neonates treated in the NICU, a total of 13 patients underwent mediastinal ultrasound for suspected abnormal radiolucency on chest radiograph. In three neonates, however, abnormal air could not be detected on ultrasound. Ultrasound findings for 10 patients (8 males and 2 females) were ultimately analyzed for this study. The age of the patients ranged from 0 to 7 days at the time of ultrasound. Birth weight ranged from 1,690 to 3,660 g with gestational age at delivery from 35 weeks 3 days to 41 weeks 2 days. Five neonates were born vaginally and five by Caesarean section. Respiratory symptoms were present in seven neonates: dyspnea in four, grunting in two, and cyanosis in one. Underlying meconium aspiration was noted in three neonates. One neonate was delivered in a car. All other neonates had no remarkable events during the delivery. Combined pneumothorax was diagnosed in three neonates. Table 1 shows the clinical characteristics and ultrasound findings of the neonates.
Using ultrasound, the mediastinal air was seen as thick linear/curvilinear echogenic lines. Some of the abnormal air accompanied posterior shadowing, while some did not. The most common sonographic finding was a thick echogenic line along the anterior margin of the thymus (Fig. 1). Air was also seen along the lateral margins of the thymus in some neonates. Less frequently, an abnormal thick echogenic line was noted between the posterior margin of the thymus and great vessels in two neonates (Fig. 2). Air was seen within thymus parenchyma in three neonates as an echogenic curvilinear lesion with posterior shadowing situated in the middle of the thymus (Fig. 3). Combined pneumothorax was seen in three neonates. Pneumomediastinum disappeared on follow-up X-ray in all patients (ranging from day 1–5). None of the neonates required drainage of the abnormal air. Two neonates later required endotracheal intubation for a short period of time due to combined pneumothorax. All other neonates were conservatively treated and discharged without any complication.
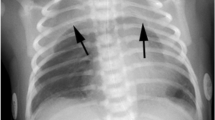
Pneumomediastinum in a 2-day-old male with dyspnea. a Chest radiograph showing abnormal radiolucency along both cardiac borders (arrows) with elevation of the thymus. b, c Transverse mediastinal ultrasound scans reveal thick echogenic lines (arrows) along the anterior and lateral borders of the thymus due to pneumomediastinum. Note that the sternum is cartilaginous, allowing transmission of the sound beam. T thymus, S sternum
Pneumomediastinum in a 0-day-old male with dyspnea. a Chest radiograph shows abnormal radiolucency (arrows) along the right border of the thymus and heart. Note deep costophrenic sulcus (arrowhead) on the right side suggesting pneumothorax. b Transverse sonogram reveals thick curvilinear echogenic lines (arrows) between the posterior margin of the thymus and great vessels due to pneumomediastinum. An echogenic curvilinear line (arrowhead) is also seen outlining the right anterior lung, probably due to combined pneumothorax. T thymus, S sternum
Pneumomediastinum in a 1-day-old male with grunting. a Chest radiograph showing abnormal radiolucency (arrows) elevating the right thymus. Note the normal wavy appearance of the left thymic border. b Transverse mediastinal sonograms reveal echogenic lines (arrows) within thymic parenchyma with posterior shadowing due to air. Note the cartilaginous sternum. c A longitudinal mediastinal sonogram showing echogenic curvilinear air (arrow) within thymic parenchyma with posterior shadowing. The heart is seen inferior to the thymus. T thymus, S sternum, H heart
In the three excluded neonates in whom abnormal air could not be detected on ultrasound, the right paracardiac radiolucency disappeared on immediate follow-up chest radiograph in two neonates. The radiolucency might have been a pseudolesion. In one neonate, pulmonary interstitial emphysema and right retrocardiac radiolucency were seen on chest radiography; however, anterior mediastinal ultrasound could not detect abnormal air.
Discussion
Pneumomediastinum is defined as the presence of air in the mediastinum. Alveolar rupture enables air to dissect through the interstitium to the hila and into the mediastinum [2]. Although pneumomediastinum is usually self-limiting, stretching of the mediastinal pleura can lead to its rupture, causing pneumothorax. Therefore, early detection of pneumomediastinum with close follow-up is needed.
The diagnosis of pneumomediastinum is generally made using frontal chest radiography. Due to the presence of the thymus, elevation of the thymus from the heart by mediastinal air can be seen as an ‘angel wing’ or ‘spinnaker sail sign,’ which is specifically present in infants. Lateral radiography is important and often more sensitive than the anteroposterior view in making the diagnosis. However, chest radiographs alone can underestimate small pneumomediastinum even if the lateral view is obtained. Chest CT scan is superior to chest radiography and can easily confirm the diagnosis even with a minimal amount of air [7]. For neonates, however, due to increased radiosensitivity and the often self-limiting nature of pneumomediastinum, chest CT is usually not performed for the purpose of merely confirming pneumomediastinum.
Chest ultrasound can be challenging due to acoustic limitations caused by aerated lung and bone, but the use of ultrasound for diagnosis of thoracic lesions has been increasing recently. The unique anatomy of the pediatric chest allows superior acoustic windows that can provide valuable information in children [5, 6]. The role of bedside sonography for trauma in emergency units has gained a well-established role in the diagnosis of pneumothorax [8].
Ultrasound is readily available, easy to perform at the bedside, and suitable for NICU. It can provide useful and important information without the need for sedation, contrast media injection, or radiation exposure. Also, ultrasound has an advantage in chest application, especially in neonates. The bony structures in the neonatal thorax are mostly cartilaginous, allowing sound beam transmission, and the thymus is relatively large, occupying most of the anterior mediastinum and not obscured by lung, allowing a good sonic view [5, 6].
Previous reports on sonographic detection of pneumomediastinum involve older children with incidental detection of air during echocardiography due to air obscuring the sonic window [9–11]. As for neonatal spontaneous pneumomediastinum, a case report of ultrasound diagnosis using a convex probe and a report of the clinical features of a series of cases have been published [3, 12]. However, no previous reports describing the detailed ultrasound features of spontaneous pneumomediastinum in NICU have been published.
Normally, a very thin echogenic line outlines the margin of the thymus and the great vessels on ultrasound. Abnormal mediastinal air was seen as echogenic thickening along the margin of the mediastinal structures, some with posterior shadowing. The most common location of abnormal air collection was along the anterior margin of the thymus. Abnormal air was also noted at the lateral margins of the thymus as well as the anterior margin in some neonates. This pattern of air collection is probably responsible for the raised thymus on simple radiographs. On chest CT of older children with extensive pneumomediastinum, the mediastinal air is usually located around the trachea and between the great vessels. A similar finding was demonstrable using ultrasound. Air was seen as a thick echogenic band between the thymus posterior margin and the great vessels. Ultrasound allowed identification of small volumes of air accumulation in this particular location, which would have been difficult to identify with simple radiography alone.
Interestingly, abnormal air situated in the middle of the thymic parenchyma was found in three neonates. This intrathymic air collection has not been previously described in the literature. The air seems to creep into the thymic tissue in certain cases, which is probably attributable to the malleable and loose nature of the thymic tissue.
Ultrasound could not detect abnormal air in one neonate with right retrocardiac radiolucency who was excluded from this study. We presume that the retrocardiac radiolucency was infra-azygos space air collection and therefore too deep to be visualized on ultrasound using a linear transducer. Depending on the location of the abnormal air, air in less common, deeper locations such as the infra-azygos space or inferior pulmonary ligament may be missed on linear ultrasound. This is a limitation of ultrasound in evaluation of abnormal air in the mediastinum. Future studies using both convex and linear probes may be helpful in such cases.
Mediastinal radiolucency is frequently encountered while interpreting NICU films, and determining the nature of this abnormal radiolucency is necessary. Although most cases of pneumomediastinum resolve spontaneously with a good clinical outcome, pneumomediastinum can lead to pneumothorax, and the neonate’s condition may rapidly deteriorate, necessitating close follow-up.
Although ultrasound cannot replace chest radiography, knowledge of the sonographic findings of pneumomediastinum can be helpful in cases where mediastinal lucency is present but the diagnosis of pneumomediastinum is not certain. The limitation of this study is that our study population involved only neonates with already-suspected abnormal air on chest radiographs. We initially intended to identify abnormal air not detectible on ultrasound, but US screening for all the neonates was ethically not possible. Further studies are needed to determine whether ultrasound can detect small pneumomediastinum that are undetectable by chest radiography and to investigate the use of ultrasound for detection of more critical mediastinal pathologies such as pneumothorax and pericardial effusion. Although preliminary in nature, this study may be helpful for wider utilization of ultrasound as a diagnostic tool for evaluating abnormal mediastinal air in NICU.
Conclusions
Ultrasound allows easy recognition of even small amounts of abnormal air in the mediastinum and gives insight into the location of abnormal air accumulation in neonatal spontaneous pneumomediastinum. Ultrasound may be used as a radiation-free adjunct imaging modality for evaluation of abnormal mediastinal radiolucency in NICU.
References
Hacking D, Stewart M. Neonatal pneumomediastinum. N Engl J Med. 2001;344:1839.
Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043–57.
Hauri-Hohl A, Baenziger O, Frey B. Pneumomediastinum in the neonatal and paediatric intensive care unit. Eur J Pediatr. 2008;167:415–8.
Greenough A, Bhojnagarwala B. Causes and management of pulmonary air leaks. Paediatr Child Health. 2012;22:523–7.
Coley BD. Chest sonography in children: current indications, techniques, and imaging findings. Radiol Clin North Am. 2011;49:825–46.
Mong A, Epelman M, Darge K. Ultrasound of the pediatric chest. Pediatr Radiol. 2012;42:1287–97.
Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration. 2000;67:408–11.
Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest. 1995;108:1345–8.
Megremis S, Stefanaki S, Tsekoura T, et al. Spontaneous pneumomediastinum in a child: sonographic detection in a case with minimal findings on chest radiography. J Ultrasound Med. 2008;27:303–6.
Testa A, Candelli M, Pignataro G, et al. Sonographic detection of spontaneous pneumomediastinum. J Ultrasound Med. 2008;27:1507–9.
Reid CL, Chandraratna AN, Kawanishi D, et al. Echocardiographic detection of pneumomediastinum and pneumopericardium: the air gap sign. J Am Coll Cardiol. 1983;1:916–21.
Van Gelderen WF. Ultrasound diagnosis of an atypical pneumomediastinum. Pediatr Radiol. 1992;22:469.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jung, A.Y., Yang, I., Go, H.S. et al. Imaging neonatal spontaneous pneumomediastinum using ultrasound. J Med Ultrasonics 41, 45–49 (2014). https://doi.org/10.1007/s10396-013-0454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-013-0454-3