Summary
Based on subgroup analyses of randomized, controlled clinical trials, we review the efficacy of three phytopharmaceutical drugs, respectively of the corresponding active substances silexan® (WS® 1265, lavender oil) in anxiety disorders, WS® 5570 (Hypericum extract) in major depression, and EGb 761® (Ginkgo biloba extract) in Alzheimer, vascular, or mixed type dementia, in elderly patients aged ≥ 60 years.
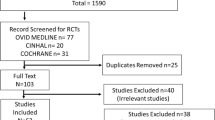
Four trials were eligible in each indication. Meta-analyses and analyses based on pooled raw data showed that the three drugs were significantly superior to placebo in the elderly subset, and that their treatment effects reflected in the main outcome measures (Hamilton Anxiety scale, Hamilton Depression scale, Neuropsychiatric Inventory) were comparable with those observed in the original trials without age restrictions.
The results confirm the efficacy of the three herbal active substances in elderly patients of ≥ 60 years of age. In anxiety, depression, and dementia, they thus represent efficacious and well-tolerated alternatives to synthetic drugs.
Zusammenfassung
Auf der Grundlage randomisierter, kontrollierter Studien untersuchten wir die Wirksamkeit dreier Phytopharmaka mit den Wirkstoffen Silexan® (WS® 1265, Lavendelöl) bei Angststörungen, WS® 5570 (Hypericum-Extrakt) bei Depressionen und EGb 761® (Ginkgo-biloba-Extrakt) bei Alzheimer- oder vaskulärer Demenz und bei Mischformen, bei Patienten im Alter ab 60 Jahren.
In jeder der drei Indikationen genügten vier Studien unseren Auswahlkriterien. Metaanalysen und Analysen auf der Grundlage der gepoolten Rohdaten wiesen auch im Kollektiv der Patienten ab 60 Jahren die Überlegenheit der drei Phytopharmaka gegenüber Plazebo nach. Dabei waren die Behandlungseffekte ähnlich jenen, die in den Ausgangsstudien für die nicht nach Alter ausgelesenen Wirksamkeitskollektive beobachtet worden waren. Das galt für die Hauptzielvariablen Hamilton Angstskala, Hamilton Depressionsskala und Neuropsychiatrisches Inventar.
Die Ergebnisse bestätigen die Wirksamkeit der drei pflanzlichen Wirkstoffe auch für ältere Patienten ab 60 Jahre. Die Präparate bieten sich damit als wirksame und gut verträgliche Alternative zu synthetischen Therapeutika bei Angststörungen, Depressionen oder Demenz an.





Similar content being viewed by others
Notes
Silexan® (WS® 1265) is the active substance of Lasea®(Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany).
WS® 5570 is the active substance of Neuroplant® (Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany).
EGb 761®is the active substance of Tebonin® (Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany).
References
European Commission. The 2015 Ageing Report: underlying assumptions and projection methodologies. In: European Economy. Brussels: European Commission (DG ECFIN) and the Economic Policy Committee (AWG); 2014.
Zimmermann T, Kaduszkiewicz H, van den Bussche H, et al. Potenziell inadaquate Medikamente bei alteren hausarztlich versorgten Patientinnen und Patienten: Eine retrospektive Langsschnittanalyse. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:941–9.
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14.
Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447–50.
Nishtala PS, Salahudeen MS. Temporal trends in polypharmacy and hyperpolypharmacy in older New Zealanders over a 9-year period: 2005–2013. Gerontology. 2014. doi:10.1159/000368191.
Pfortmueller CA, Lindner G, Exadaktylos AK. Reducing fall risk in the elderly: risk factors and fall prevention, a systematic review. Minerva Med. 2014;105:275–81.
Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing. 2015;44:90–6.
Hein C, Forgues A, Piau A, et al. Impact of polypharmacy on occurrence of delirium in elderly emergency patients. J Am Med Dir Assoc. 2014;15:850e11–5.
Leiss W, Mean M, Limacher A, et al. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J Gen Intern Med. 2015;30:17–24.
Polypharmazie TPA. Treiben Sie den Teufel nicht mit dem Beelzebub aus. MMW Fortschr Med. 2014;156:56–61 (quiz 2).
Gómez C, Vega-Quiroga S, Bermejo-Pareja F, et al. Polypharmacy in the elderly: a marker of increased risk of mortality in a population-based prospective study (NEDICES). Gerontology. 2014. doi:10.1159/000365328.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65.
Wickop B, Langebrake C. Gute Verordnungspraxis bei älteren Patienten. Ther Umsch. 2014;71:366–73.
Loew D. Pflanzliche versus synthetische Arzneimittel in der Geriatrie—eine Betrachtung zur Arzneimittelsicherheit aus pharmakologischer und pharmakokinetischer Sicht. Ars Medici Thema Phytotherapie. 2012:15–20.
Loew D, Beer AM, Adler M. Phytotherapie in der Praxis: Einsatzmöglichkeiten bei geriatrischen Erkrankungen. Z Phytother. 2010;31:145–8.
Sami MB, Nilforooshan R. The natural course of anxiety disorders in the elderly: a systematic review of longitudinal trials. Int Psychogeriatr. 2014. doi:10.1017/S1041610214001847.
Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77–84.
Council of Europe. European Pharmacopoeia. 8th ed. Strasbourg: Council of Europe; 2015.
Schuwald AM, Nöldner M, Wilmes T, et al. Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PloS one. 2013;8:e59998.
Baldinger P, Hoflich AS, Mitterhauser M, et al. Effects of Silexan on the serotonin-1 A receptor and microstructure of the human brain: a randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int J Neuropsychopharmacol. 2014. doi:10.1093/ijnp/pyu063.
Kasper S. An orally administered lavandula oil preparation (Silexan) for anxiety disorder and related conditions: an evidence based review. Int J Psychiatry ClinPract. 2013;17(Suppl. 1):15–22.
Kasper S, Gastpar M, Müller WE, et al. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: a randomized, double-blind, placebo controlled trial. Int Clin Psychopharmacol. 2010;25:277–87.
Kasper S, Gastpar M, Müller WE, et al. Lavender oil preparation Silexan is effective in generalized anxiety disorder—a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacolog. 2014;17:859–69.
Woelk H, Schläfke S. A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine. 2010;17:94–9.
Hamilton M. Hamilton Anxiety Scale (HAMA). In: Guy W, editor, ECDEU assessment manual for psychopharmacology. Rockville: U.S. National Institute of Health, Psychopharmacology Research Branch; 1976.pp 193–7.
Kasper S, Anghelescu I, Dienel A. Efficacy of Silexan (WS® 1265) in patients with restlessness and sleep disturbance. In: Annual Congress of the German Society for Psychiatry and Psychotherapy (DGPPN). Berlin, Germany; 2010.
National Institute of Mental Health. 12– CGI. Clinical Global Impressions. In: Guy W, editor, EDCEU Assessment in Psychopharmacology. Rockville: U.S. National Institute of Mental Health, Psychopharmacology Research Branch; 1970. pp 217–22.
Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113:372–87.
Rodda J, Walker Z, Carter J. Depression in older adults. BMJ. 2011;343:d5219.
Helmchen H, Linden M, Wernicke T. Psychiatrische Morbidität bei Hochbetagten. Ergebnisseaus der Berliner Altersstudie. Nervenarzt. 1996;67:739–50.
Linden M, Kurtz G, Baltes MM, et al. Depression bei Hochbetagten. Ergebnisse der Berliner Altersstudie. Nervenarzt. 1998;69:27–37.
Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551.
Bauer M, Pfennig A, Severus E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334–85.
Müller WE. Current St John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47:101–9.
Wurglics M, Westerhoff K, Kaunzinger A, et al. Comparison of German St. John’s wort products according to hyperforin and total hypericin content. J Am Pharm Assoc (Wash). 2001;41:560–6.
Gastpar M. Hypericum extract WS (R) 5570 for depression—an overview. Int J Psychiatry Clin Pract. 2013;17(Suppl. 1):1–7.
Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258:59–63.
Kasper S, Gastpar M, Möller HJ, et al. Better tolerability of St. John’s wort extract WS 5570 compared to treatment with SSRIs: a reanalysis of data from controlled clinical trials in acute major depression. Int Clin Psychopharmacol. 2010;25:204–13.
Linde K. St.John’s wort—an overview. Forsch Komplementarmed. 2009;16:146–55.
Borrelli F, Izzo AA. Herb-drug interactions with St John’s wort (Hypericumperforatum): an update on clinical observations. AAPS J. 2009;11:710–27.
Lecrubier Y, Clerc G, Didi R, et al. Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry. 2002;159:1361–6.
Kasper S, Anghelescu I, Szegedi A, et al. Superior efficacy of St John’s wort extract WS® 5570 compared to placebo in patients with major depression: a randomized, double-blind, placebo-controlled, multi-center trial. BMC Med. 2006. doi:10.1186/1741-7015-4-14.
Szegedi A, Kohnen R, Dienel A, et al. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine. BMJ. 2005;330:503–6.
Kasper S, Volz HP, Möller HJ, et al. Continuation and long-term maintenance treatment with Hypericum extract WS 5570 after recovery from an acute episode of moderate depression—a double-blind, randomized, placebo controlled long-term trial. Eur Neuropsychopharmacol. 2008;18:803–13.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96.
Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement. 2013;9:63–75 e2.
Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32.
Berr C, Wancata J, Ritchie K. Prevalence of dementia in the elderly in Europe. Eur Neuropsychopharmacol. 2005;15:463–71.
Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204.
Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–81.
Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51.
Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12:2–32.
Abdel-Kader R, Hauptmann S, Keil U, et al. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761). Pharmacol Res. 2007;56:493–502.
Költringer P, Langsteger W, Eber O. Dose-dependent hemorheological effects and microcirulatory modifications following intravenous administration of Ginkgo biloba special extract EGb 761. Clin Hemorheol. 1995;15:649–56.
Ramassamy C, Longpre F, Christen Y. Ginkgo biloba extract (EGb 761) in Alzheimer’s disease: is there any evidence? Curr Alzheimer Res. 2007;4:253–62.
Tchantchou F, Xu Y, Wu Y, et al. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–8.
Wu Y, Wu Z, Butko P, et al. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26:13102–13.
Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9:2065–77.
Ihl R. Effects of Ginkgo biloba extract EGb 761 (R) in dementia with neuropsychiatric features: review of recently completed randomised, controlled trials. Int J Psychiatry Clin Pract. 2013;17(Suppl. 1):8–14.
Janßen IM, Sturtz S, Skipka G, et al. Ginkgo biloba in Alzheimer’s disease: a systematic review. Wien Med Wochenschr. 2010;160:539–46.
Tan MS, Yu JT, Tan CC, et al. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2015;43:589–603.
Yang M, Xu DD, Zhang Y, et al. A systematic review on natural medicines for the prevention and treatment of Alzheimer’s disease with meta-analyses of intervention effect of ginkgo. Am J Chin Med. 2014;42:505–21.
Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–6.
Herrschaft H, Nacu A, Likhachev S, et al. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46:716–23.
Ihl R, Bachinskaya N, Korczyn AD, et al. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:1186–94.
Napryeyenko O, Borzenko I;Gindem-Np Study Group. Ginkgo biloba special extract in dementia with neuropsychiatric features.A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11.
Nikolova G, Yancheva S, Raychev I, et al. Gingo biloba extract in dementia: a 22-week randomised, placebo-controlled, double-blind trial. Bulg Neurol. 2013;14:139–43.
Schneider G, Heuft G. Angst und Depression beiälteren Menschen. Z Psychosomatische Med Psychother. 2012;58:336–56.
Forsell Y, Jorm AF, von Strauss E, et al. Prevalence and correlates of depression in a population of nonagenarians. Br J Psychiatry. 1995;167:61–4.
Trindade E, Menon D, Topfer LA, et al. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. Can Med Assoc J. 1998;159:1245–52.
Montgomery SA. A meta-analysis of the efficacy and tolerability of paroxetine versus tricyclic antidepressants in the treatment of major depression. Int Clin Psychopharmacol. 2001;16:169–78.
Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. 2014;371:1228–36.
Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? Eur Neuropsychopharmacol. 1999;9(Suppl. 6):S399–405.
Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8:45–58.
Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60:753–9.
Muliyala KP, Varghese M. The complex relationship between depression and dementia. Ann Indian Acad Neurol. 2010;13:S69–S73.
Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205.
Barry LC, Abou JJ, Simen AA, et al. Under-treatment of depression in older persons. J Affect Disord. 2012;136:789–96.
Licht-Strunk E, Van Marwijk HW, Hoekstra T, et al. Outcome of depression in later life in primary care: longitudinal cohort study with three years’ follow-up. BMJ. 2009;338:a3079.
Nutt D, Allgulander C, Lecrubier Y, et al. Establishing non-inferiority in treatment trials in psychiatry: guidelines from an Expert Consensus Meeting. J Psychopharmacol. 2008;22:409–16.
Masson SC, Tejani AM. Minimum clinically important differences identified for commonly used depression rating scales. J Clin Epidemiol. 2013;66:805–7.
Furukawa TA, Akechi T, Azuma H, et al. Evidence-based guidelines for interpretation of the Hamilton rating scale for depression. J Clin Psychopharmacol. 2007;27:531–4.
Kim YS, Nibbelink DW, Overall JE. Factor structure and scoring of the SKT test battery. J Clin Psychol. 1993;49:61–71.
Ihl R, Grass-Kapanke B, Lahrem P, et al. Entwicklung und Validierungeines Tests zur Früherkennung der Demenz mit Depressionsabgrenzung (TFDD). Fortschr Neurol Psychiatr. 2000;68:413–22.
Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37:725–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Herrn Prof. Hippius zum 90. Geburtstag gewidmet.
Rights and permissions
About this article
Cite this article
Kasper, S. Phytopharmaceutical treatment of anxiety, depression, and dementia in the elderly: evidence from randomized, controlled clinical trials. Wien Med Wochenschr 165, 217–228 (2015). https://doi.org/10.1007/s10354-015-0360-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-015-0360-y