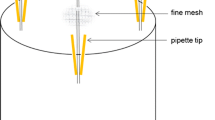
Abstract
Aphid parasitoids are commonly used in the biological control of aphids. However, their success in biological control largely depends on the availability of carbohydrate-rich food as an energy source for maintenance and reproduction. Therefore, as these resources have become rare in modern agricultural systems, external sugar sources like flowering plants or artificial sugar solutions are more and more used to provide the biocontrol agents with the necessary sugars. When developing such artificial food sources, it is essential to carefully select the sugars that best support the target parasitoids without benefiting non-target insects, such as pest insects or hyperparasitoids. Here, we investigated the gustatory response and longevity of two commonly used aphid parasitoids (Aphidius colemani and Aphidius matricariae) and their hyperparasitoid Dendrocerus aphidum when provided with one of eight plant- and/or insect-derived sugars (fructose, galactose, glucose, melibiose, melezitose, rhamnose, sucrose and trehalose). Our results showed that the studied insect species consumed the largest amounts of sugars that are most commonly found in honeydew (sucrose, fructose, glucose and melezitose) and also survived best when feeding on these sugars. Both Aphidius spp. survived well on melibiose, whereas D. aphidum performed poorly on this sugar. When melibiose was offered in a mixture with glucose, a significant reduction in longevity was observed for D. aphidum when compared to glucose only, while this was less pronounced for Aphidius spp. This knowledge can be exploited in tailoring food sources to selectively support Aphidius parasitoids, enhancing the biological control of aphids.


Similar content being viewed by others
References
Araj SE, Wratten S, Lister A, Buckley H (2006) Floral nectar affects longevity of the aphid parasitoid Aphidius ervi and its hyperparasitoid Dendrocerus aphidum. New Zeal Plant Prot 59:178–183
Araj SE, Wratten S, Lister A, Buckley H (2008) Floral diversity, parasitoids and hyperparasitoids–A laboratory approach. Basic Appl Ecol 9:588–597
Araj SE, Wratten S, Lister A, Buckley H (2009) Adding floral nectar resources to improve biological control: potential pitfalls of the fourth trophic level. Basic Appl Ecol 10:554–562
Bass C, Puinean AM, Zimmer CT, Denholm I, Field LM, Foster SP, Gutbrod O, Nauen R, Slater R, Williamson MS (2014) The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem Mol Biol 51:41–51
Beach PJ, Williams L, Hendrix DL, Pice LD (2003) Different food sources affect the gustatory response of Anaphes iole, an egg parasitoid of Lygus spp. J Chem Ecol 29:1203–1222
Benelli G, Giunti G, Tena A, Desneux N, Caselli A, Canale A (2017) The impact of adult diet on parasitoid reproductive performance. J Pest Sci. doi:10.1007/s10340-017-0835-2
Campbell AJ, Biesmeijer JC, Varma V, Wäckers FL (2012) Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic Appl Ecol 13:363–370
Carter C, Shafir S, Yehonatan L, Palmer R, Thornburg R (2006) A novel role for proline in plant floral nectars. Naturwissenschaften 93:72–79
Charles JJ, Paine TD (2016) Fitness effects of food resources on the polyphagous aphid parasitoid, Aphidius colemani Viereck (Hymenoptera: Braconidae: Aphidiinae). PLoS ONE 11:e0147551. doi:10.1371/journal.pone.0147551
Dedryver C, Le Ralec A, Fabre F (2010) The conflicting relationships between aphids and men: a review of aphid damage and control strategies. C R Biol 333:539–553
Fischer MK, Shingleton AW (2001) Host plant and ants influence the honeydew sugar composition of aphids. Funct Ecol 15:544–550
Fischer MK, Voelkl W, Hoffmann KH (2005) Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera: Aphididae) on various host plants and implications for ant-attendance. Eur J Entomol 102:155–160
Gómez-Marco F, Urbaneja A, Jaques JA, Rugman-Jones PF, Stouthamerc R, Tena A (2015) Untangling the aphid-parasitoid food web in citrus: can hyperparasitoids disrupt biological control? Biol Control 81:111–121
Hagvar EB, Hofsvang T (1991) Aphid parasitoids (Hymenoptera: Aphidiidae): biology, host selection and use in biological control. Biocontrol News Inf 12:13–41
Harvey JA, Cloutier J, Visser B, Ellers J, Wäckers FL, Gols R (2012) The effect of different dietary sugars and honey on longevity and fecundity in two hyperparasitoid wasps. J Insect Physiol 58:816–823
Heimpel GE, Jervis MA (2005) Does floral nectar improve biological control by parasitoids. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 267–304
Hillocks RJ (2012) Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot 31:85–93
Hogervorst PA, Romeis J, Wäckers FL (2003) Suitability of honeydew from potato infesting aphids as food source for Aphidius ervi. Proc Exp Appl Entomol 14:87–90
Hogervorst PA, Wäckers FL, Romeis J (2007) Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol Exp Appl 122:223–232
Höller C, Borgemeister C, Haardt H, Powell W (1993) The relationship between primary parasitoids and hyperparasitoids of cereal aphids: an analysis of field data. J Anim Ecol 62:12–21
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105
Jones DB, Giles KL, Berberet RC, Royer TA, Elliott NC, Payton ME (2003) Functional response of an introduced parasitoid and an indigenous parasitoid on greenbug at four temperatures. Environ Entomol 32:425–432
Lenaerts M, Abid L, Paulussen C, Goelen T, Wäckers F, Jacquemyn H, Lievens B (2016) Adult parasitoids of honeydew-producing insects prefer honeydew sugars to cover their energetic needs. J Chem Ecol 42:1028–1036
Mackauer M, Völkl W (1993) Regulation of aphid populations by aphidiid wasps: does parasitoid foraging behaviour or hyperparasitism limit impact? Oecologia 94:339–350
Nault LR (1997) Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am 90:521–541
R Core Team (2014) A language and environment for statistical com-puting. In: Cowles RS, Saona CR, Holdcraft R, Loeb GM, Elsensohn Foundation for statistical computing: Vienna
Shimoda T, Mitsunaga T, Uefune M, Abe J, Kugimiya S, Nagasaka K, Sano K, Urano S, Suzuki Y, Yano E, Takabayashi J (2014) A food-supply device for maintaining Cotesia vestalis, a larval parasitoid of the diamondback moth Plutella xylostella, in greenhouses. Biocontrol 59:681–688
Snyder WE, Ives AR (2003) Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84:91–107
Sullivan DJ, Völk W (1999) Hyperparasitism: multitrophic ecology and behavior. Ann Rev Entomol 44:291–315
Tena A, Llácer E, Urbeneja A (2013a) Biological control of a non-honeydew producer mediated by a distinct hierarchy of honeydew quality. Biol Control 67:117–122
Tena A, Pekas A, Wäckers FL, Urbaneja A (2013b) Energy reserves of parasitoids depend on honeydew from non-hosts. Ecol Entomol 38:278–289
Tena A, Pekas A, Cano D, Wäckers FL, Urbeneja A (2015) Sugar provisioning maximizes the biocontrol service of parasitoids. J Appl Ecol 52:795–804
van Emden HF, Harrington R (2007) Aphids as crop pests. CABI, London
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20
van Lenteren JC, Woets J (1988) Biological and integrated pest control in greenhouses. Annu Rev Entomol 33:239–269
van Rijn PCJ, Wäckers FL (2016) Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J Appl Ecol 53:925–933
Wäckers FL (1999) Gustatory response by the hymenopteran parasitoid Cotesia glomerata to a range of nectar and honeydew sugars. J Chem Ecol 25:2863–2877
Wäckers FL (2001) A comparison of nectar-and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata. J Insect Physiol 47:1077–1084
Wäckers FL (2005) Suitability of (extra-)floral nectar, pollen, and honeydew as insect food sources. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 267–304
Wäckers FL, van Rijn PCJ (2012) Pick and mix: selecting flowering plants to meet the requirements of target biological control insects. In: Gurr GM, Wratten SD, Snyder WE, Read DMY (eds) Biodiversity and insect pests: key issues for sustainable management. Wiley, Chichester, pp 139–165
Walker GP, Cameron PJ (1981) The biology of Dendrocerus carpenteri (Hymenoptera: Ceraphronidae), a parasite of Aphidius species, and field observations of Dendrocerus species as hyperparasites of Acyrthosiphon species. New Zeal J Zool 8:531–538
Williams L, Roane TM (2007) Nutritional ecology of a parasitic wasp: food source affects gustatory response, metabolic utilization, and survivorship. J Insect Physiol 53:1262–1275
Winkler K, Wäckers FL, Stingli A, van Lenteren JC (2005) Plutella xylostella (diamondback moth) and its parasitoid Diadegma semiclausum show different gustatory and longevity responses to a range of nectar and honeydew sugars. Entomol Exp Appl 115:187–192
Winkler K, Wäckers F, Bukovinszkine-Kissa G, van Lenteren JC (2006) Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl Ecol 7:133–140
Yano E (2006) Ecological considerations for biological control of aphids in protected culture. Popul Ecol 48:333–339
Acknowledgements
We are grateful to the Flemish Fund for Scientific Research (FWO) for supporting this research. Additionally, we would like to thank all PME & BIM colleagues who helped during the course of the experiments.
Funding
This work was supported by the Flemish Fund for Scientific Research (FWO) (TG is a SB PhD fellow at FWO (project 1S15116316 N)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
This article does not contain studies with humans participants performed by any of the authors.
Additional information
Communicated by N. Agusti.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goelen, T., Baets, D., Kos, M. et al. Gustatory response and longevity in Aphidius parasitoids and their hyperparasitoid Dendrocerus aphidum . J Pest Sci 91, 351–360 (2018). https://doi.org/10.1007/s10340-017-0907-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0907-3