Abstract
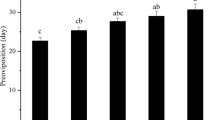
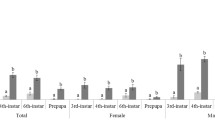
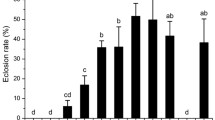
Bactrocera minax is a major citrus pest in China, Bhutan, and India. It is univoltine and exhibits pupal diapause during winter. To better understand pupal diapause in this pest, we investigated pupal survival and pupal developmental duration under field and laboratory conditions. Specifically, we tested if pupal chilling was required for diapause development and termination. Nearly all mature larvae collected at the end of the citrus season entered pupal diapause. For pupae exposed in the field, natural chilling for less than 3 months resulted in more than 70 % mortality. However, exposure to winter conditions for 3 months or more both decreased pupal mortality and developmental duration when pupae were returned to the laboratory and held under constant temperature (25 °C). When pupae were gathered from the field in November and exposed to different chilling regimes in the laboratory, the chilling duration (30 vs 60 days) had significantly more impact on pupal survival than the specific chilling temperature (6, 8, 10, or 12 °C constant). However, both chilling duration and chilling temperature impacted on the pupal developmental duration, with longer chilling duration and higher temperatures decreasing pupal developmental duration. In conclusion, we demonstrated that pupal diapause development and termination in B. minax is strongly influenced by chilling conditions. Increasing cold exposure led to significantly and consistently faster adult eclosion and improved synchronization of adult emergence. This knowledge will help with the laboratory rearing of B. minax, an essential step in the long-term management of this pest.





Similar content being viewed by others
References
Baerwald RJ, Boush MG (1967) Selection of a nondiapausing race of apple maggot. J Econ Entomol 60:682–684
Baird CR (1972) Termination of pupal diapause in Cuterebra tenebrosa (Diptera: Cuterebridae) with injections of ecdysterone. J Med Entomol 9:77–80
Bosch J, Kemp WP (2003) Effect of wintering duration and temperature on survival and emergence time in males of the orchard pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environ Entomol 32:711–716
Canale A, Benelli G (2012) Impact of mass-rearing on the host seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szepligeti) (Hymenoptera: Braconidae). J Pest Sci 85:65–74
Collier RH, Elliott MS, Finch S (1994) Development of the overwintering stages of the carrot fly, Psila rosae (Diptera: Psilidae). Bull Entomol Res 84:469–476
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
Dambroski HR, Feder JL (2007) Host plant and latitude-related diapause variation in Rhagoletis pomonella: a test for multifaceted life history adaptation on different stages of diapause development. J Evol Biol 20:2101–2112. doi:10.1111/j.1420-9101.2007.01435.x
Danks H (2007) The elements of seasonal adaptations in insects. Can Entomol 139:1–44
Denlinger DL (2002) Regulation of diapause. Ann Rev Entomol 47:93–122
Dorji C, Clarke AR, Drew RAI, Fletcher BS, Loday P, Mahat K, Raghu S, Romig MC (2006) Seasonal phenology of Bactrocera minax (Diptera: Tephritidae) in western Bhutan. Bull Entomol Res 96:531–538
Fan JA, Zhao XQ, Zhu J (1994) A study on the cold-resistance and diapause in Tetradacus citri Chen. J Southwest Agric Univ 16:532–534
Fujiwara Y, Denlinger DL (2007) High temperature and hexane break pupal diapause in the flesh fly, Sarcophaga crassipalpis, by activating ERK/MAPK. J Insect Physiol 53:1276–1282
Goncalves MF, Torres LM (2011) The use of the cumulative degree-days to predict olive fly, Bactrocera oleae (Rossi), activity in traditional olive groves from the northeast of Portugal. J Pest Sci 84:187–197
Han P, Wang X, Niu C, Dong Y, Zhu J, Desneux N (2011) Population dynamics, phenology, and overwintering of Bactrocera dorsalis (Diptera: Tephritidae) in Hubei Province, China. J Pest Sci 84:289–295
Hodek I (1996) Diapause development, diapause termination and the end of diapause. Eur J Entomol 93:475–488
Hodek I (2002) Controversial aspects of diapause development. Eur J Entomol 99:163–174
Kidokoro K, Wata K, Fujiwara Y, Takeda M (2006) Effects of juvenile hormone analogs and 20-hydroxyecdysone on diapause termination in eggs of Locusta migratoria and Oxya yezoensis. J Insect Physiol 52:473–479
Kostal V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52:113–127
Kostal V, Havelka J (2001) Low temperature storage of larvae and synchronization of adult emergence in the predatory midge Aphidoletes aphidimyza. Cryobiology 42:112–120. doi:10.1006/cryo.2001.2311
Kostal V, Shimada K, Hayakawa Y (2000) Induction and development of winter larval diapause in a drosophilid fly, Chymomyza costata. J Insect Physiol 46:417–428
Krafsur ES (1998) Sterile insect technique for suppressing and eradicating insect populations: 55 years and counting. J Agr Entomol 15:303–317
Kuriwada T, Kumano N, Shiromoto K, Haraguchi D (2011) Pre-exposure to sex pheromone did not affect mating behavior in the sweetpotato weevil Cylas formicarius. J Pest Sci 84:93–97
Lauzon CR, Potter SE (2012) Description of the irradiated and nonirradiated midgut of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha ludens Loew (Diptera: Tephritidae) used for sterile insect technique. J Pest Sci 85:217–226
Lv ZZ (2006) Eclosion, mating and oviposition behavior of the Chinese citrus fly, Bactrocera minax (Enderlein), in Yichang region. Plant Quar 20:215–216
Moraiti CA, Nakas CT, Papadopoulos NT (2012) Prolonged pupal dormancy is associated with significant fitness cost for adults of Rhagoletis cerasi (Diptera: Tephritidae). J Insect Physiol 58:1128–1135
Moribe Y, Niimi T, Yamashita O, Yaginuma T (2001) Samui, a novel cold-inducible gene, encoding a protein with a BAG domain similar to silencer of death domains (SODD/BAG-4), isolated from Bombyx diapause eggs. Eur J of Biochem 268:3432–3442
Nomura M, Ishikawa Y (2000) Biphasic effect of low temperature on completion of winter diapause in the onion maggot, Delia antiqua. J Insect Physiol 46:373–377
Ragland GJ, Fuller J, Feder JL, Hahn DA (2009) Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J Insect Physiol 55:344–350
Shan Y (2008) Present situation, development trend and countermeasures of citrus industry in China. J Chin Inst Food Sci Technol 8:1–8
Smith SL, Jones VP (1991) Alteration of apple maggot (Diptera: Tephritidae) emergence by cold period duration and rain. Environ Entomol 20:44–47
Stross R (1966) Light and temperature requirements for diapause development and release in Daphnia. Ecology 47:368–374
Tang S, Gong QT, Dou W, Wang JJ, Zhao ZM (2012) Effects of temperature, soil humidity and depth of buried pupae on adult emergence of Bactrocera minax. Acta Phytophy Sinica 39:137–141
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford, p 411
Teixeira LAF, Polavarapu S (2001) Postdiapause development and prediction of emergence of female blueberry maggot (Diptera: Tephritidae). Environ Entomol 30:925–931
Teixeira LAF, Polavarapu S (2002) Phenological differences between populations of Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 31:1103–1109
Teixeira LAF, Polavarapu S (2005a) Diapause development in the blueberry maggot Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 34:47–53
Teixeira LAF, Polavarapu S (2005b) Heat stress inhibits the completion of pupal diapause in Rhagoletis mendax (Diptera: Tephritidae). Ann Entomol Soc Am 98:197–204
Teixeira LAF, Polavarapu S (2005c) Expression of heat shock protein 70 after heat stress during pupal diapause in Rhagoletis mendax (Diptera: Tephritidae). Ann Entomol Soc Am 98:966–972
Teixeira LAF, Polavarapu S (2005d) Evidence of a heat-induced quiescence during pupal development in Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 34:292–297
Terao M, Hirose Y, Shintani Y (2012) Effects of temperature and photoperiod on termination of pseudopupal diapause in the bean blister beetle Epicauta gorhami. J Insect Physiol. doi:10.1016/j.jinsphys.2012.02.009
Turnock W, Lamb R, Bodnaryk R (1983) Effects of cold stress during pupal diapause on the survival and development of Mamestra configurata (Lepidoptera: Noctuidae). Oecologia 56:185–192
Vankirk JR, Aliniazee MT (1982) Diapause developmnet in the western cherry fruit fly, Rhagoletis indifferens Curran (Diptera, Tephridae). J Appl Entomol 93:440–445
Wang X, Luo L (1995) Research progress in the Chinese citrus fruit fly. Entomol Knowl 32:310–315
White IM, Wang X (1992) Taxonomic notes on some dacine (Diptera: Tephritidae) fruit flies associated with citrus, olives and cucurbits. Bull Entomol Res 82:275–279
Xu WH, Lu YX, Denlinger DL (2012) Cross-talk between the fat body and brain regulates insect developmental arrest. Proc Natl Acad Sci USA. doi:10.1073/pnas.1212879109
Yasuda T, Narahara M, Tanaka S, Wakamura S (1994) Thermal responses in the citrus fruit fly, Dacus tsuneonis: evidence for a pupal diapause. Entomol Exp Appl 71:257–261
Zdarek J, Denlinger DL (1975) Action of ecdysoids, juvenoids, and non-hormonal agents on termination of pupal diapause in the flesh fly. J Insect Physiol 21:1193–1202
Zhang Q, Nachman RJ, Kaczmarek K, Zabrocki J, Denlinger DL (2011) Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc Natl Acad Sci USA 108(41):16922–16926
Zhou XW, Niu CY, Han P, Desneux N (2012) Field evaluation of attractive lures for the fruit fly Bactrocera minax (Diptera: Tephritidae) and their potential use in spot sprays in Hubei Province (China). J Econ Entomol 105(4):1277–1284
Acknowledgments
We thank David Denlinger and Vladimír Koštál for valuable suggestions on the manuscript. We also acknowledge three anonymous reviewers for their constructive comments, which help us to improve the quality of this manuscript. This study was financially supported by the Fundamental Research Funds for the Central Universities (2011PY055), National Science Foundation of China (No. 31071690) and the International Atomic Energy Agency (via Research Contract No. 16015 to C.N. and Expert Mission Contract CPR/5/020-01-01 to A.R.C.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Dong, YC., Wang, ZJ., Clarke, A.R. et al. Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax . J Pest Sci 86, 429–436 (2013). https://doi.org/10.1007/s10340-013-0493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0493-y