Abstract
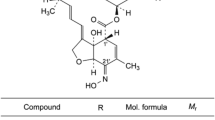
Two sensitive and selective stability-indicating methods were developed for the determination of the antibiotic cefpirome sulfate in bulk powder, pharmaceutical formulation and in presence of its acid, alkaline, photo- and oxidative degradation products. Method A was based on HPLC separation of cefpirome sulfate in the presence of its degradation products on a reversed phase column C18, 250 × 4.6 mm, 5-μm particle size and mobile phase consisting of 0.1 M disodium hydrogen phosphate dihydrate pH 3.9 adjusted with phosphoric acid–acetonitrile (85:15, v/v). Quantitation was achieved with UV detection at 270 nm. The linear calibration curve was in the range 5.0–50.0 μg mL−1. Method B was based on reversed phase TLC separation of the cited drug in the presence of its degradation products followed by densitometric measurement of the intact drug at 270 nm. The separation was carried out using disodium hydrogen phosphate dihydrate 2.0 g %w/v, at pH 3.5 adjusted with phosphoric acid–acetone (15:10, v/v) as a developing system. The calibration curve was in the range of 1.0–10.0 μg/spot. The HPLC method was used to study the kinetic of cefpirome sulfate acid degradation. The results obtained were statistically analyzed and compared with those obtained by applying the official Japanese method.








Similar content being viewed by others
References
The Japanese Pharmacopoeia (2006) 15th edition English Version, Tokyo
Sweetman S (2011) Martindale “The extra pharmacopoeia”, The Complete Drug Reference 37th edition, The Pharmaceutical Press, London
Turley C, Kearns G, Jacobos R (1988) Micro-analytical HPLC assay for cefpirome (HR810) in serum. Antimicrob Agen Chemoth 32(10):1481–1483
Sugioka T, Asano T, Chikaraishi Y, Suzuki E, Sano A, Kuriki T, Shirotsuka M, Satio K (1990) Stability and degradation pattern of cefpirome (HR 810) in aqueous solution. Chem Pharm Bull 38(7):1998–2002
Nahata M (1991) Determination of cefpirome in human plasma by HPLC. J Liq Chromatogr 14(1):193–200
Tabata S, Koike Y, Sato Y, Kobayashi I, Hirayama M, Hasegawa Y (1991) Determination method and stability of cefpirome sulfate in biological samples. Chemoth Tokyo 39(1):92–99
Kearns G, Johnson V, Hendry I, Wells T (1992) Microanalytical HPLC assay for cefpirome in human milk and urine. J Chromatogr 574(2):356–360
Pehourcq F, Jarry C (1998) Determination of third-generation cephalosporins by high performance liquid chromatography in connection with pharmacokinetic studies. J Chromatogr A 812(1–2):159–178
Breilh D, Lavallee C, Fratta A, Ducint D, Cony-Maklouf P, Saux M (1999) Determination of cefepime and cefpirome in human serum by high-performance liquid chromatography using an ultrafiltration for antibiotics serum extraction. J Chromato B., Biomed Sci Appl 734(1):121–127
Cherti N, Kinowski J, Lerfant J, Bressolle F (2001) High-performance liquid-chromatographic determination of cefepime in human plasma and in urine and dialysis fluid using a column-switching technique. J Chromato B Biomed Sci Appl 754(2):377–386
Arayne M, Sultana N, Nawaz M (2008) Simultaneous quantification of cefpirome and cetirizine or levocetirizine in pharmaceutical formulations and human plasma by RP-HPLC. J Anal Chem 63(9):881–887
Rao K, Kumar K, Joydeep D (2011) New stability indicating RP-HPLC method for the estimation of cefpirome sulphate in bulk and pharmaceutical dosage forms. Sci Pharm 79:899–907
Dhanesar S (1999) Quantitation of antibiotics by densitometry on a hydrocarbon-impregnated silica gel HPTLC plate, part III: quantitation and evaluation of cephalosporins. J Plan Chromato Mod TLC 12(2):114–119
Mayer B, Hollenstein U, Brunner M, Eichler H, Mueller M (2000) Micellar electrokinetic chromatography for the analysis of cefpirome in microdialysis and plasma samples obtained in vivo from human volunteers. Electrophoresis 21(8):1558–1564
Prakash K, Rao J, Raju N, Himabindu V (2008) Spectrophotometric estimation of cefpirome sulfate in pharmaceutical formulations. Asian J Chem 20(4):2587–2590
Evagelou V, Tsantili-Kakoulidou A, Koupparis M (2003) Determination of the dissociation constants of the cephalosporins, cefepime and cefpirome using UV spectrometry and pH potentiometry. J Pharm Biomed Anal 31(6):1119–1128
National Committee for Clinical Laboratory Standards (1993) Performance standards for antimicrobial disc susceptibility tests. Approved standard NCCLS publication M2-A5. Villanova, USA
International Conference of Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Definitions and Terminology, Stability Testing of New Drug Substances and Products Q1A (R2) (2003) Geneva
Sainsbury M (2001) Heterocyclic chemistry. The Royal Society of Chemistry, UK, pp 18–22
Begtrup M, Hansen L (1992) New methods for the introduction of substituents into thiazole. Acta Chem Scand 46:372–383
The United States Pharmacopeia and National Formulary. USP 34-NF 29 (2011) US pharmacopeial convention. Rockville, USA
Florence A, Attwood D (1998) Physical principles of pharmacy, 2nd edn. Macmillan, London
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elghobashy, M.R., Bebawy, L.I., Abbas, S.S. et al. Stability-Indicating HPLC and RP-TLC Determination of Cefpirome Sulfate with Kinetic Study. Chromatographia 76, 1141–1151 (2013). https://doi.org/10.1007/s10337-013-2516-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2516-5