Abstract
A rapid, precise, accurate, and selective high-performance liquid chromatographic method with fluorescence detection has been validated and used for analysis of amisulpride in human plasma after a simple solid-phase extraction procedure. Compounds were separated on a CN column with 0.03 M potassium dihydrogen phosphate (pH 6.5)–acetonitrile 65:35 (v/v) as mobile phase. Fluorescence detection was performed at excitation and emission wavelengths of 274 and 370 nm, respectively. Calibration plots were linear over the concentration range 10–1,000 ng mL−1 in human plasma, and the lower limit of quantification was 10 ng mL−1. Accuracy was between 0.4 and 6.4% and precision was between 3.1 and 7.5%. Amisulpride was sufficiently stable through three freeze–thaw cycles, during storage for 6 h at room temperature, and for 2 months at −22 °C. The method is suitable for the analysis of clinical samples from pharmacokinetic studies.
Similar content being viewed by others
Introduction
Amisulpride ((R,S)-4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulphonyl-2-methoxybenzamide) is a 2-methoxybenzamide derivative chemically related to sulpiride. It is an antagonist of dopamine D2 and D3 receptors [1]. It is effective as an antidysthymic, antischizophrenic, or antipsychotic drug, depending on the dose: 50–100 mg for treatment of dysthymic disorders [2, 3], over 200 mg for treatment of negative symptoms in schizophrenic patients, and over 600 mg for psychotic disorders [4].
Amisulpride is rapidly absorbed, with an absolute bioavailability of approximately 50% and a biphasic absorption profile [5, 6]. Two distinct plasma concentration peaks are usually observed approximately 1 and 3–4 h (T max) after administration. The protein binding of this drug is approximately 17% and the average amisulpride elimination half-life from plasma, after oral administration, is approximately 11–12 h [5, 6]. Low metabolism (approx. 5% of the administered dose) is a characteristic of amisulpride, and unmodified drug is primarily excreted in the urine (50% of an intravenous dose is excreted via the urine, of which 90% is eliminated in the first 24 h) [4, 5].
Several methods have been used for analysis of amisulpride in human plasma, including gas chromatography [7], radioreceptor assay [8], and high-performance liquid chromatography (LC) with UV [9–11], mass spectrometric [12, 13], or fluorescence [14, 15] detection.
Gschwend et al. [13] reported a sensitive LC method with mass spectrometric detection after liquid–liquid extraction (diisopropyl ether–dichloromethane 1:1 (v/v)); a low limit of quantification (LLOQ) of 0.5 ng mL−1 amisulpride was achieved. Kirchherr et al. [12] used another LC–MS method for analysis of antidepressants and achieved an LLOQ of 28 ng mL−1 for amisulpride.
Frahnert et al. [9] used solid-phase extraction for isolation of amisulpride from plasma. Chromatographic separation on a Nucleosil 100-Protect 1 column was followed by UV detection. Response was a linear function of concentration over the range 10–1,000 ng mL−1.
A fully automated chromatographic method including on-line blood serum or plasma clean-up, isocratic LC, and spectrophotometric detection was reported by Sachse et al. [10]. Plasma or serum was cleaned on a silica CN (10 × 4.0 mm i.d., 20-μm particle) column and further separation was performed on a LiChrospher CN column. The limit of detection was 10 ng mL−1 and the linear range 10–600 ng mL−1.
Malavasi et al. [14] used both liquid–liquid extraction and solid-phase extraction for isolation of amisulpride from human plasma. LC separation was performed on a Hypersil C18 BDS column. Fluorescence detection with excitation and emission wavelengths of 280 and 370 nm, respectively, resulted in a limit of quantification of 0.5 ng mL−1 and a calibration range of 0.5–640 ng mL−1.
The objective of this work was to develop and validate a rapid and sensitive LC method with simple sample preparation for analysis of amisulpride in human plasma, and to use it in a randomized cross-over bioequivalence study after oral administration of two different amisulpride preparations (200 mg) to 18 healthy volunteers. Fluorescence detection was chosen as more sensitive and specific than UV detection. Most existing methods for analysis of amisulpride in human plasma have an upper limit of quantification in the range 600–640 ng mL−1. This is suitable for study of pharmacokinetics after oral administration of 50 mg amisulpride. After an oral dose of 200 mg the expected C max is >500 ng mL−1 with a standard deviation of approximately 200 ng mL−1, and thus the upper limit of quantification should be not less than 850 ng mL−1. Our method includes a simple single-stage SPE procedure (loading–washing–collection). The method was validated in accordance with FDA guidelines [16] to assess its selectivity, sensitivity, accuracy, and precision.
Experimental
Chemicals, Reagents, and Solutions
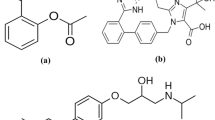
The amisulpride standard was a European Pharmacopoeia standard (purity 99.9%; Council of Europe, European Directorate for the Quality of Medicines and Health Care, Strasbourg, France). Metoclopramide hydrochloride (4-amino-5-chloro-N-[(2-diethylamino)ethyl]-2-methoxybenzamide hydrochloride; purity 99.73%), used as internal standard (IS), was purchased from Ipca Laboratories (Mumbai, India). The chemical structures of both substances are shown in Fig. 1.
Methanol and acetonitrile were HPLC-grade (Sigma–Aldrich, St Louis, MO, USA). Triethylamine, orthophosphoric acid (85%), and potassium dihydrogen phosphate were analytical grade (Fluka, Buchs, Switzerland). Double-distilled water purified with a Milli-Q5 system (Millipore, Bedford, MA, USA) was used in all experiments.
Human plasma was obtained from the Kharkov Center of Blood Service (Kharkov, Ukraine).
Oasis HLB solid-phase extraction cartridges (1 cc, 30 mg; Waters, Milford, MA, USA) were used for sample preparation.
Amisulpride stock standard solution (1 mg mL−1) was prepared in methanol. Working solutions (1.0, 2.5, 3.0, 5.0, 10, 25, 30, 50, 75, 90, and 100 μg mL−1) were prepared by dilution of the stock solution with pure water. These amisulpride working solutions were used for preparation of validation plasma samples (standard calibration samples and quality-control samples) and amisulpride working standard solutions.
Amisulpride working standard solutions were prepared at concentrations of 20, 50, 100, 200, 500, 1,000, 1,500, and 2,000 ng mL−1, by dilution of 200 μL of the appropriate amisulpride working solution to 10 mL with water–acetonitrile 75:25 (v/v). Amisulpride working standard solutions were used for assessment of the linearity of detector response.
Internal standard (metoclopramide) solution (1 mg mL−1) was prepared in pure water and diluted to 100 μg mL−1 with pure water.
To prepare calibration standards, 2 mL human plasma was mixed with 20 μL of the appropriate amisulpride working solution to furnish concentrations of 10, 25, 50, 100, 250, 500, 750, and 1,000 ng mL−1. The IS solution (100 μg mL−1, 15 μL) was added to 1.5 mL of the corresponding calibration standard and the procedure for sample preparation, described below, was carried out.
To prepare quality-control samples 250 μL of the appropriate amisulpride working solution was diluted with 25 mL human plasma to furnish concentrations of 30, 300, and 900 ng mL−1.
To prepare amisulpride samples with concentrations above the calibration range (1,800 ng mL−1), 500 μL amisulpride working solution (concentration 90 μg mL−1) was diluted with 25 mL human plasma. This solution was used to test the integrity of the dilution procedure.
IS solution (100 μg mL−1, 15 μL) was added to 1.5 mL of the corresponding validation sample and the procedure for sample preparation, described below, was carried out.
Sample Preparation
Frozen plasma samples were thawed at ambient temperature (20 ± 2 °C) and vortex-mixed for 30 s. Plasma (1.5 mL) was placed in a plastic tube, 15 μL IS solution (100 μg mL−1) was added, and vortex mixing was performed for 30 s. Samples were centrifuged at 11,000g for 15 min. After centrifugation, 1.0 mL of the supernatant was loaded on to a previously activated SPE cartridge. The cartridge was washed with 1 mL pure water then the amisulpride and metoclopramide were eluted with 1.0 mL methanol. The eluent was collected in a polypropylene tube and evaporated to dryness under vacuum at 58 °C. The residue was reconstituted with 500 μL water–acetonitrile mixture 75:25 (v/v) and the solution was vortex-mixed and analyzed.
Instrumentation and Chromatographic Conditions
HPLC was performed with a Perkin Elmer (Shelton, USA) series 200 system consisting of vacuum degasser, gradient pump, thermostatic autosampler, column oven, fluorescence detector, diode-array detector, and network chromatographic interface (NCI 900). TotalChrom ver. 6.0 software (Perkin Elmer) was used for acquisition and processing of chromatographic data.
Chromatographic separation was performed at 35 °C on a 250 × 4.6 mm i.d., particle size 5 μm, Zorbax SB-CN analytical column (Agilent Technologies, USA) with a 12.5 × 4.6 mm i.d., particle size 5 μm, Zorbax SB-CN guard column (Agilent). The mobile phase was 0.03 M phosphate buffer (pH 6.5)–acetonitrile 65:35 (v/v). The 0.03 M phosphate buffer solution (pH 6.5) was prepared by dissolving 4.1 g potassium dihydrogen phosphate in 950 mL pure water, adjusting to pH 6.5 ± 0.1 with triethylamine, and diluting to 1,000 mL with pure water. The mobile phase flow-rate was 1 mL min−1 and the injection volume was 40 μL. Fluorescence detection (excitation and emission wavelengths 274 and 370 nm, respectively) was used for detection of amisulpride and the IS.
Optimization of SPE
Solid-phase extraction (SPE) was chosen for sample preparation. This method enables simultaneous and rapid processing of many samples, cleaning extracts from biological material and concentration of determined compounds.
Oasis HLB cartridges were chosen for extraction of amisulpride and the internal standard from plasma samples. These cartridges contain adsorbent with both hydrophobic and hydrophilic properties. Preliminary sample extraction conditions were selected by using aqueous solutions the compounds investigated. The amisulpride and IS content of washings from the cartridge were determined after each stage of sample preparation (sorption, washing, and collection). Sample-preparation conditions were the same as those proposed by the cartridge manufacturer: conditioning (pre-washing) with 1 mL methanol then 1 mL water, washing with water and 30% (v/v) methanol–water, then collection by use of 1 mL methanol. Amisulpride and the IS were not found in the washing liquids after the adsorption and washing stages. The methanolic sample solution obtained in the collection stage was dried under vacuum and the residue was re-dissolved in 1.0 mL water–acetonitrile 75:25 (v/v). The amisulpride and IS content were approximately 90 and 80%, respectively, of the theoretical values (determined by analysis of solutions containing the compounds without the sample-preparation stage).
After development, sample extraction was performed on plasma containing known concentrations of analyte and IS. Endogenous plasma components did not interfere with the compounds of interest after their isolation by use of SPE. Pure acetonitrile, a mixture of equal volumes of acetonitrile and methanol, and double the volume of methanol were used as liquids for collection of amisulpride and IS but, because these solvents did not lead to more efficient extraction, 1 mL methanol was chosen for collection.
Optimization of LC
High-performance liquid chromatography with different methods of detection have been used for pharmacokinetic studies of amisulpride. We compared the sensitivity for amisulpride using the same chromatographic conditions but with application of diode-array and fluorescence detection. The DAD was set at the wavelength of maximum absorption, 274 nm, and fluorescence detection was performed with excitation and emission wavelengths of 274 and 370 nm, respectively. It was established that under similar chromatographic conditions fluorescence detection was six times more sensitive that UV detection.
Because the CN stationary phase used is more polar than C8 and C18 it enables faster separation with low mobile phase organic modifier content.
In bioanalysis, LC retention of the analytes should be sufficient to avoid interferences with the first peak in the chromatogram. For this reason, the effect of mobile phase pH on analyte retention was studied. Mobile phase pH tested was 6.0, 6.5, and 7.0. It was found that retention of amisulpride in this pH range did not vary significantly. Use of an acidic (pH 3.0) mobile phase, however, substantially reduced retention and resolution of amisulpride and the IS. Reduction of the acetonitrile content led to increased retention time and resolution of amisulpride and IS, but separation of the analyte from endogenous components of the plasma was not achieved. The pH eventually selected was 6.5.
To achieve good retention of amisulpride and good resolution between amisulpride and IS the effect of the acetonitrile content of the mobile phase was studied. The retention time of amisulpride and amisulpride–IS resolution were determined using acetonitrile concentrations ranging from 25 to 50% (v/v). The retention times and resolution of the analytes decreased as the concentration of organic modifier was increased (Table 1). The final acetonitrile concentration selected was 35% (v/v).
Results and Discussion
Validation
The method was validated in accordance with USP 32 [17], International Conference of Harmonization (ICH) Guidelines [16, 18, 19], and other literature [20].
Selectivity
Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components of the sample; selectivity should be ensured at the lower limit of quantification (LLOQ). Six blank plasma samples from different sources were used for evaluation of method selectivity. Blank samples were tested for interference with the analyte and the IS. The selectivity criterion was that the maximum interference observed in blank plasma samples should not exceed one-fifth of the analyte response at the LLOQ [16].
Chromatograms obtained from blank human plasma, plasma spiked with amisulpride (10 ng mL−1; LLOQ) and metoclopramide (IS, 1,000 ng mL−1) and from plasma spiked with amisulpride (1,000 ng mL−1; upper limit of quantification, ULOQ) and the IS are presented in Fig. 2, from which it apparent no interference was detected. The spectral purity of the peaks of amisulpride and the IS was, furthermore, monitored by use of the DAD and found to be more than 99% for both at the ULOQ level.
Linearity
The linearity of detector response was estimated by analysis of working standard solutions of amisulpride in the concentration range 20–2,000 ng mL−1. The dependence of detector response (peak area) on amisulpride concentration was described by the equation Y = 2060.3C + 998.26, r = 0.9996 (where Y is peak area, C is amisulpride concentration in ng mL−1, and r is the correlation coefficient).
The linearity of the method was evaluated by constructing a calibration plot in the range 10–1,000 ng mL−1 amisulpride in plasma by plotting the amisulpride-to-internal standard peak-area ratio against amisulpride concentration. Least-squares linear regression analysis with the weighting coefficient 1/C 2 was used to determine the relationship between amisulpride concentration and detector response.
Each day the slope, intercept, and correlation coefficient were determined; results of these investigations are presented in Table 2. The resulting calibration equation was Y = (18.3 ± 0.7) × 10−4 C + (6 ± 15) × 10−4, r = 0.9979; the relationship between peak-area ratio and amisulpride concentration was linear within the concentration range studied.
The limit of detection (LOD) of amisulpride in plasma samples was 2 ng mL−1 (the serial dilution method was used; signal-to-noise ratio = 3). The lowest limit of quantification (LLOQ) was defined as the lowest concentration in the linear region of the calibration plot for which the coefficient of variation (CV) was reproducibly below 20% and accuracy between 80 and 120%. The average LLOQ from five determinations was 10 ng mL−1.
Accuracy and Precision
Accuracy and precision were determined by replicate analysis of samples with known amisulpride content. The mean value should be within 15% of the actual value [16, 20, 21]. The difference between mean amounts of amisulpride added and recovered (RE, %) serves as a measure of accuracy. The coefficient of variation (CV, %), as a measure of precision at each concentration, should not exceed 15%.
Intra-day and inter-day accuracy and precision were evaluated by analysis of quality-control samples containing amisulpride at three different concentrations—a low concentration (LQC), 30 ng mL−1, a concentration near the centre of the calibration plot (MQC), 300 ng mL−1, and a concentration near the upper limit of the calibration plot (HQC), 900 ng mL−1. Intra-day accuracy and precision were evaluated by analysis of these QC samples prepared and analyzed on 1 day (eight samples of each concentration; three replicate injections). Inter-day accuracy and precision were evaluated by analysis of these QC samples prepared and analyzed on five different days (three samples of each concentration; three replicate injections).
The dilution procedure for plasma samples containing amisulpride concentrations outside the calibration range was validated by analysis of eight diluted samples with an amisulpride concentration of 1,800 ng mL−1 (2 × HQC), and evaluation of accuracy and precision from the results obtained.
The intra-day and inter-day precision and accuracy of the method are presented in Table 3. Intra-day precision ranged between 3.13 and 5.35% and inter-day precision was between 4.54 and 7.50%. Intra-day accuracy was between −3.00 and 6.41% and inter-day accuracy was between 0.40 and 4.11%. Precision and accuracy of analysis of diluted samples were also satisfactory.
All values for accuracy and precision were within the recommended limits.
Recovery
Recovery of amisulpride and the IS from plasma was determined at concentrations of 30, 300, and 900 ng mL−1 for amisulpride and 1,000 ng mL−1 for metoclopramide by comparing peak areas obtained from plasma with those obtained from the same amounts of unextracted solutions [16]. Recovery was 94% for amisulpride and 95% for metoclopramide.
Stability
The stability of amisulpride in human plasma over three freeze–thaw cycles and during short-term, long-term, and post-preparative storage was tested by analysis of LQC (30 ng mL−1) and HQC (900 ng mL−1) samples [16].
The freeze–thaw stability of the amisulpride was determined over three freeze–thaw cycles within 3 days. Spiked plasma samples were frozen at −22 °C for 24 h and thawed at room temperature in each freeze–thaw cycle. To study short-term stability, the frozen (−22 °C) and then thawed plasma samples were kept at room temperature for 6 h before sample preparation. The postpreparative stability of prepared plasma samples was studied after keeping the samples in the autosampler at 16 °C for 48 h. The results obtained from these test samples were compared with those from freshly thawed and processed samples (reference samples).
Long-term stability was determined after keeping spiked plasma samples frozen at −22 °C for 2 months. For this stability test the samples (test samples) were analyzed and the results were compared with those obtained from freshly prepared and processed samples (reference samples) [22].
The stability of amisulpride and metoclopramide (IS) in stock solutions was studied after storage at 2 °C for 1 month.
The results obtained from assessment of stability are given in Table 4. Three freeze–thaw cycles of the quality-control samples did not seem to affect quantification of the amisulpride. Quality-control samples stored in a freezer at −22 °C were stable for at least 2 months. Thawing of the frozen samples and keeping them at room temperature for 6 h had no effect on quantification. Prepared samples stored in autosampler at 16 °C were stable for at least 48 h.
The stability of amisulpride and metoclopramide in stock solutions was confirmed after storage for 29 days at 2 °C.
Bioequivalence Study
The applicability of the method was demonstrated in a randomized crossover bioequivalence study of two amisulpride preparations—Solian 200 mg (Sanofi Winthrop Industrie, Gentille, France), used as the reference preparation, and Soleron 200 (Pharma Start, Kyiv, Ukraine) the investigated preparation.
Eighteen healthy volunteers (ten male and eight female), age 26–30 years, weight 53–85 kg, participated in the study. The subjects were underwent a clinical examination, electrocardiogram, and laboratory tests. The investigation was approved by the Ethics Committee and written informed consent was obtained from all the subjects. No volunteer took any drug for at least 14 days before the study or during it.
The volunteers were randomly divided into two groups of nine. The study was divided into two periods with a 6-day washout period. In the first period, group 1 received the reference preparation and group 2 received the test preparation. A crossover design was used in the study: in the second period, group 1 received the test preparation and group 2 the reference preparation.
A single oral dose of amisulpride (200 mg) was administrated under fasting conditions and serial blood samples were collected at suitable intervals up to 36 h. Pharmacokinetic data including the area under the concentration–time curve during the sampling period (AUC 0–36), the area under the concentration–time curve from zero to infinity (AUC 0–∞), and elimination half life (T 1/2) were calculated by use of WinNonLin v. 5.2 (Pharsight, USA) pharmacokinetics software. The maximum plasma concentration (C max) and the time to reach the peak plasma concentration (T max) were obtained directly from the individual drug plasma concentration–time plots. A paired t test was used for statistical evaluation of the data and all statistical calculations were defined at the level P ≤ 0.05. Typical pharmacokinetic profiles (plasma concentration–time) for the two amisulpride preparations are presented in Fig. 3 and the main pharmacokinetic data are summarized in Table 5.
Conclusion
A simple, accurate, rapid, and selective reversed-phase LC method with fluorescence detection for analysis of amisulpride in human plasma after its isolation by solid-phase extraction has been developed and validated. A one-step solid-phase extraction procedure is proposed for preparation of plasma samples for analysis of amisulpride during pharmacokinetic investigations.
The sensitivity of the method (LLOD) was almost the same as that described in the literature for analogous pharmacokinetic studies, but in this method the amisulpride ULOQ was increased substantially—up to 1,000 ng mL−1—because the orally administered dose was increased to 200 mg compared with 50 mg investigated previously, and because of the need to perform pharmacokinetic investigations with this pharmaceutical preparation (Soleron 200).
The method was successfully applied to a bioequivalence study of two amisulpride preparations after oral administration of a 200-mg dose to eighteen healthy volunteers. It was demonstrated that both amisulpride preparations had similar pharmacokinetic properties.
References
Coukell AJ, Spencer CM, Benfield P (1996) CNS Drugs 6:237–243
Costa JA, Silva E (1990) Ann Psychiatr 5:242–256
Agnoli A, Ravizza L, Torta R (1989) In: Proc 37th Italian Psychiatry Society Congress, Rome, 6–11 February 1989, CIC International, Rome
Josserand F, Weber F (1988) Ann Psychiatr 3:306–312
Noble S, Benfield P (1999) CNS Drugs 12:471–477
Hamon-Vilcot B, Chaufour S, Deschamps C, Canal M, Zieleniuk I, Ahtoy P, Chretien P, Rosenzweig P, Nasr A, Piette F (1998) Eur J Clin Pharmacol 54:405–415
Kamizono A, Inotsume N, Miyamote K, Ueda K, Miyakawa T, Arimoto H, Nakano M (1991) J Chromatogr B 567:113–119
Mokrim R, Brunet C, Cazin M, Gressier B, Luyckx M, Dine T, Robert H, Cazin JC (1993) Methods Find Exp Clin Pharmacol 15:41–50
Frahnert C, Rao ML, Grasmäder K (2003) J Chromatogr B 794:35–40. doi:10.1016/S1570-0232(03)00393-3)
Sachse J, Härtter S, Weigmann H, Hiemke C (2003) J Chromatogr B 784:405–411. doi:10.1016/S1570-0232(02)00808-5
Pehourcq F, Ouariki S, Begaud B (2003) J Chromatogr B 789:101–117. doi:10.1016/S1570-0232(03)00045-X
Kirchherr H, Kühn-Velten WN (2006) J Chromatogr B 843:100–105. doi:10.1016/j.jchromb.2006.05.031
Gschwend MH, Arnold P, Ring J, Martin W (2006) J Chromatogr B 831:132–140. doi:10.1016/j.jchromb.2005.11.042
Malavasi B, Locatell M, Ripamonti M, Ascalone V (1996) J Chromatogr B 676:107–112
Ascalone V, Ripamonti M, Malavasi B (1996) J Chromatogr B 676:95–101
Guidance for industry, bioanalytical method validation. US Department of Heaths and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Rockville, MD, 2001. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm
United States Pharmacopoeia 32/National Formulary 27 (2009) United State Pharmacopoeial Convention. Rockville, MD, pp 733–740
International Conference of Harmonization, Q2A: Text on validation of analytical procedures. (1995) US FDA Federal Register, V. 60, March, pp 11260–11273
International Conference of Harmonization, Q2B: Validation of analytical procedures: methodology. (1997) US FDA Federal Register, V. 62, May, pp 27463–27495
Bansal S, DeStefano A (2007) AAPS J 9:109–115. doi:10.1208/aapsj0901011
Viswanathan C, Bansal S, Booth B, DeStefano A, Rose M, Sailstad J, Shah V, Skelly J, Swann P, Weiner R (2007) AAPS J 9:30–38. www.aapsj.org (doi:10.1208/aapsj0901004)
Nowatzke W, Woolf E (2007) AAPS J 9:117–122. doi:10.1208/aapsj0902013
Acknowledgments
This work was supported by Pharma Start Ltd, (Kyiv, Ukraine), sponsor of the bioequivalence study of two amisulpride preparations (Solian 200 mg, Sanofi Winthrop Industrie S.A., Gentille, France and Soleron 200, Pharma Start, Kyiv, Ukraine).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kudris, I.V., Skakun, N.N., Orlova, I.N. et al. Analysis of Amisulpride in Human Plasma by SPE and LC with Fluorescence Detection. Chromatographia 73, 67–74 (2011). https://doi.org/10.1007/s10337-010-1832-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-010-1832-2