Abstract
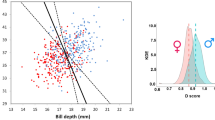
Assortative mating is an important aspect of mate choice, especially in species where both sexes express ornamentation. Such ornaments could function as signals of individual quality and could result in individuals mating with partners of similar quality. We tested for assortative mating by measuring 63 pairs of Atlantic Puffins (Fratercula arctica) at two Canadian colonies (Gull Island, Witless Bay, Newfoundland and Labrador; and Machias Seal Island, New Brunswick), and constructed a function to predict the sex of puffins from Witless Bay. Male and female puffins have similar plumage, and both sexes have fleshy rosettes at the base of their bill, which are supposedly ornaments. We also examined changes in measurements over time in 5–30-year-old puffins recaptured at Machias Seal Island. Our discriminant function correctly predicted the sex of 88 % of puffins from Witless Bay. Overall, males were larger than females in all measurements, but within pairs, some females were larger in 4–27 % of individual measurements. We found no evidence of positive assortative mating or of assortative mating by rosette size, and rosette area did not increase with age. The importance of puffins’ rosettes as indicators of quality requires further investigation.
Zusammenfassung
Geschlechtsbezogener Größendimorphismus und Diskriminanzfunktionen zur Geschlechtsbestimmung bei Papageitauchern ( Fratercula arctica )
Assortative Paarung ist ein wichtiger Aspekt der Partnerwahl. Das gilt besonders für Arten, bei denen beide Geschlechter ornamentale Merkmale aufweisen. Solche Ornamente könnten als Signale individueller Qualität fungieren und dazu führen, dass Individuen sich mit Partnern ähnlicher Qualität verpaaren. Wir untersuchten die Frage assortativer Paarung, indem wir 63 Papageitaucherpaare (Fratercula arctica) aus zwei kanadischen Kolonien (Gull Island, Witless Bay, Neufundland und Labrador sowie Machias Seal Island, New Brunswick) vermaßen, und entwickelten eine Funktion zur Vorhersage des Geschlechts bei den Papageitauchern von Witless Bay. Bei Papageitauchern haben Männchen und Weibchen ähnliches Gefieder und beide Geschlechter tragen an der Schnabelbasis fleischige Rosetten, die vermutlich ornamentale Funktion besitzen. Außerdem betrachteten wir die zeitlichen Veränderungen der Maße bei 5–30 Jahre alten Papageitauchern, die auf Machias Seal Island wiedergefangen wurden. Unsere Diskriminanzfunktion konnte bei 88 % der Papageitaucher von Witless Bay das Geschlecht korrekt vorhersagen. Generell waren die Männchen in allen Maßen größer als die Weibchen, innerhalb der Paare waren allerdings manche Weibchen in 4–27 % der Einzelmaße größer. Es gab keine Belege für eindeutig assortative Paarung. Es gab keine Hinweise auf assortative Paarung anhand der Rosettengröße; die Rosettenfläche nahm mit dem Alter auch nicht zu. Die Bedeutung der Rosetten als Qualitätsmerkmale bei Papageitauchern bedarf weiterer Untersuchungen.


Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson M, Iwasa Y (1996) Sexual selection. Trends Ecol Evol 11:53–58
Anker-Nilssen T, Aarvak T, Bangjord G (2003) Mass mortality of Atlantic Puffins Fratercula arctica off central Norway, spring 2002: causes and consequences. Atl Seabirds 5:57–71
Arnqvist G, Mårtensson T (1998) Measurement error in geometric morphometrics: empirical strategies to assess and reduce its impact on measures of shape. Acta Zool Acad Sci Hung 44:73–96
Baker AJ (1974) Criteria for aging and sexing New Zealand oystercatchers. N Z J Mar Freshw Res 8:211–221
Baldwin SP, Oberholder HC, Worley LG (1931) Measurements of birds. Sci Publ Cleveland Mus Nat Hist 2:1–165
Barrett RT, Fieler R, Anker-Nilssen T, Rikardsen F (1985) Measurements and weight changes of Norwegian adult puffins Fratercula arctica and kittiwakes Rissa tridactyla during the breeding season. Ringing Migr 6:102–112
Barrett RT, Nilsen EB, Anker-Nilssen T (2012) Long-term decline in egg size of Atlantic puffins Fratercula arctica is related to changes in forage fish stocks and climate conditions. Mar Ecol Prog Ser 457:1–10
Bates D, Machler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv: http://arxiv.org/abs/1406.5823
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Berzins LL, Gilchrist HG, Burness G (2009) No assortative mating based on size in Black Guillemots breeding in the Canadian Arctic. Waterbirds 32:459–463
Bond AL, Jones IL, Seneviratne SS, Muzaffar SB (2013) Least Auklet (Aethia pusilla). In: Poole A (ed) The birds of North America, No. 69. Retrieved from the Birds of North America Online. http://bna.birds.cornell.edu/bna/species/069. Cornell Lab of Ornithology, Ithaca, NY
Burger AE (1980) Sexual size dimorphism and aging characters in the Lesser Sheathbill at Marion Island. Ostrich 51:39–51
Cohen J (1960) A coefficient of agreement for nominal states. Educ Psychol Measur 20:37–46
Corkhill P (1972) Measurements of puffins as criteria of age and sex. Bird Study 19:193–201
Creelman E, Storey AE (1991) Sex differences in reproductive behaviour of Atlantic Puffins. Condor 93:390–398
Crespi BJ (1989) Assortative mating in arthropods. Anim Behav 38:980–1000
Cuervo JJ, de Lope F, Møller AP (1996) The function of long tails in female Barn Swallows (Hirundo rustica): an experimental study. Behav Ecol 7:132–136
Darwin CR (1871) The descent of man, and selection in relation to sex. John Murray, London
De Marchi G, Fasola M, Chiozzi G, Bellati A, Galeotti P (2012) Sex discrimination of Crab Plovers (Dromas ardeola) by morphometric traits. Waterbirds 35:332–337
Devlin CM, Diamond AW, Saunders GW (2004) Sexing Arctic Terns in the field and laboratory. Waterbirds 27:314–320
Douletrent C, Grégoire A, Gomez D, Staszewski V, Arnoux E, Tveraa T, Faivre B, Boulinier T (2013) Colouration in Atlantic Puffins and Black-legged Kittiwakes: monochromatism and links to body condition in both sexes. J Avian Biol 44:451–460
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases, and sexual selection. Trends Ecol Evol 13:415–420
Fairbairn DJ (2007) Introduction: the enigma of sexual size dimorphism. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual dimorphism. Oxford University Press, Oxford, pp 1–10
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Fletcher KL, Hamer KC (2003) Sexing terns using biometrics: the advantage of within-pair comparisons. Bird Study 50:78–83
Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertelloti M, Blanco G (2002) Food resource utilization by the Magellanic Penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299
Friars KA, Diamond AW (2011) Predicting the sex of Atlantic Puffins, Fratercula arctica, by discriminant analysis. Waterbirds 34:304–311
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
García LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663
Gonzalez-Solis J (2004) Sexual size dimorphism in northern giant petrels: ecological correlates and scaling. Oikos 105:247–254
Grecian VD, Diamond AW, Chardine JW (2003) Sexing Razorbills Alca torda breeding at Machias Seal Island, New Brunswick, Canada, using discriminant function analysis. Atl Seabirds 5:73–80
Hallgrimsson GT, Palsson S, Summers RW (2008) Bill length: a reliable method for sexing Purple Sandpipers. J Field Ornithol 79:87–92
Harris MP (1979) Measurements and weights of British Puffins. Bird Study 26:179–186
Harris MP (2014) Aging Atlantic Puffins Fratercula arctica in summer and winter. Seabird 27:22–40
Harris MP, Wanless S (2011) The Puffin. T & AD Poyser, London
Jakubas D, Wojczulanis K (2007) Predicting the sex of Dovekies by discriminant analysis. Waterbirds 30:92–96
Johnstone RA (1997) The tactics of mate choice and competitive search. Behav Ecol Sociobiol 40:51–59
Johnstone RM, Niven BE (1989) Sexing Grey-faced Petrels by discriminant analysis of measurements. Notornis 36:261–265
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1381–1391
Jones IL (1993) Sexual differences in bill shape and external measurements of Crested Auklets. Wilson Bull 105:525–529
Jones IL, Hunter FM (1993) Mutual sexual selection in a monogamous seabird. Nature 362:238–239
Jones IL, Hunter FM (1999) Experimental evidence for mutual inter- and intrasexual selection favouring a Crested Auklet ornament. Anim Behav 57:521–528
Jones IL, Hunter FM, Robertson GJ, Fraser GS (2004) Natural variation in the sexually selected feather ornaments of Crested Auklets (Aethia cristatella) does not predict future survival. Behav Ecol 15:332–337
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philos Trans R Soc Lond B Biol Sci 357:319–330
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic chatacters. Evolution 24:292–305
Levene H (1960) Robust tests for equality of variances. In: Olkin I (ed) Contributions to probability and statistics: essays in honor of Harold Hotelling. Stanford University Press, Stanford, pp 278–292
Lowther PE, Diamond AW, Kress SW, Robertson GJ, Russell K (2002) Atlantic Puffin (Fratercula arctica). In: Poole A, Gill F (eds) The birds of North America, No. 709. Philadelphia: The Birds of North America, Inc.
Ludwig SC, Becker PH (2008) Supply and demand: causes and consequences of assortative mating in Common Terns Sterna hirundo. Behav Ecol Sociobiol 62:1601–1611
Mawhinney K, Diamond AW (1999) Sex determination of Great Black-backed Gulls using morphometric characters. J Field Ornithol 70:206–210
Meiri S, Dayan T (2003) On the validity of Bergmann’s rule. J Biogeogr 30:331–351
Moen SH (1991) Morphologic and genetic variation among breeding colonies of the Atlantic Puffin (Fratercula arctica). Auk 108:755–763
Møller AP, Jennions MD (2001) How important are direct fitness benefits of sexual selection. Naturwissenschaften 88:401–415
Murphy TG (2008) Lack of assortative mating for tail, body size, or condition in the elaborate monomorphic Turquoise-browed Motmot (Eumomota superciliosa). Auk 125:11–19
Murphy TG, Pham TT (2012) Condition and brightness of structural blue-green: motmot tail-racket brightness is related to speed of feather growth in males, but not in females. Biol J Linn Soc 106:673–681
Nelson DA (1981) Sexual differences in measurements of Cassin’s Auklet. J Field Ornithol 52:233–234
Nisbet ICT, Bridge ES, Szczys P, Heidinger BJ (2007) Sexual dimorphism, female–female pairs, and test for assortative mating in Common Terns. Waterbirds 30:169–179
Nordeide JT, Kekälälnen J, Janhunen M, Kortet R (2013) Female ornaments revisited—are they correlated with offspring quality? J Anim Ecol 82:26–38
Palestris BG, Nisbet ICT, Hatch JJ, Arnold JM, Szczys P (2012) Tail length and sexual selection in a monogamous, monomorphic species, the Roseate Tern Sterna dougallii. J Ornithol 153:1153–1163
Peck DR, Congdon BC (2006) Sex-specific chick provisioning and diving behaviour in the Wedge-tailed Shearwater Puffinus pacificus. J Avian Biol 37:245–251
Perktaş U, Gosler AG (2010) Measurement error revisited: its importance for the analysis of size and shape of birds. Acta Ornithol (Wars) 45:161–172
Pradhan GR, Van Schaik CP (2009) Why do females find ornaments attractive? The coercion-avoidance hypothesis. Biol J Linn Soc 96:372–382
R Core Team (2014) R: a language and environment for statistical computing. Version 3.1.2 [computer program]. R Foundation for Statistical Computing, Vienna, Austria
Ripley B, Venables B, Hornik K, Gebhardt A, Firth D (2012) MASS–Functions and datasets to support Venables and Ripley, ‘Modern Applied Statistics with S’ (4th edition, 2002). R package version 7.3-23. http://CRAN.R-project.org/package=MASS
Robinson SA, Forbes MR, Hebert CE, Scheuhammer AM (2011) Evidence for sex differences in mercury dynamics in Double-crested Cormorants. Environ Sci Technol 45:1213–1218
Ryan MJ (1998) Sexual selection, receiver biases, and the evolution of sex differences. Science 281:1999–2003
Sandvik H (2001) Sexing animals using biometry: intra-pair comparison is often superior to discriminant functions. Fauna Nor 21:11–16
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Sheridan JA, Bickford D (2011) Shrinking body size as an ecological response to climate change. Nat Clim Change 1:401–406
Székely T, Lislevand T, Figuerola J (2007) Sexual size dimorphism in birds. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual dimorphism. Oxford University Press, Oxford, pp 27–37
Tarvin KA, Murphy TG (2012) It is not always sexy when both are bright and shiny: considering alternatives to sexual selection in elaborate monomorphic species. Ibis 154:439–443
Tomassini A, Colangelo P, Agnelli P, Jones G, Russo D (2014) Cranial size has increased over 133 years in a common bat, Pipistrellus kuhlii: a response to changing climate or urbanization? J Biogeogr 41:944–953
van de Pol M, Verhulst S (2006) Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am Nat 167:764–771
van de Pol M, Wright J (2009) A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 77:753–758
van Rooij EP, Griffith SC (2012) No evidence of assortative mating on the basis of putative ornamental traits in long-tailed Finches Peophila acuticauda. Ibis 154:444–451
Wagner RH (1999) Sexual size dimorphism and assortative mating in Razorbills (Alca torda). Auk 116:542–544
Welcker J, Steen H, Harding AMA, Gabrielsen GW (2009) Sex-specific provisioning behaviour in a monomorphic seabird with a bimodal foraging strategy. Ibis 151:502–513
Wolf WL, Casto JM, Nolan V Jr, Ketterson ED (2006) Female ornamentation and male mate choice in Dark-eyed Juncos. Anim Behav 67:93–102
Yezerinac SM, Lougheed SC, Handford P (1992) Measurement error and morphometric studies: statistical power and observer experience. Syst Biol 41:471–482
Acknowledgments
We thank D.W. Pirie-Hay, M. Ballasteros, A.K. Bowser, J. Davey, C. Jardine, K.G. Kelly, B. Martin, D. Ogden, E. Tompkins, and E. Whidden for assistance in the field. The Parks and Natural Areas Division, Newfoundland and Labrador Department of Environment and Conservation kindly granted permission for our work in the Witless Bay Ecological Reserve; the Canadian Coast Guard and Canadian Wildlife Service granted permission to work on Machias Seal Island. A. Patterson (Bold Coast Charter Company, Cutler, Maine), G.J. Robertson, S.I. Wilhelm, and Environment Canada provided logistic support. B. Pilgrim and E. Perry performed the genetic analysis, and S.L. Van Wilgenburg provided statistical advice. The Canadian Wildlife Service (permit SC 2783) and University of Saskatchewan Animal Research Ethics Board (protocol 20120008) approved this research. The Natural Sciences and Engineering Research Council of Canada and Environment Canada provided financial support. Comments from G.J. Robertson and two anonymous reviewers improved previous drafts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Krüger.
Rights and permissions
About this article
Cite this article
Bond, A.L., Standen, R.A., Diamond, A.W. et al. Sexual size dimorphism and discriminant functions for predicting the sex of Atlantic Puffins (Fratercula arctica). J Ornithol 157, 875–883 (2016). https://doi.org/10.1007/s10336-016-1332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1332-8