Abstract
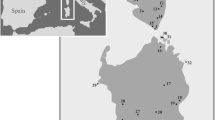
We have used coalescent analysis of mtDNA cytochrome b (cyt b) sequences to estimate times of divergence of three species of Alouatta—A. caraya, A. belzebul, and A. guariba—which are in close geographic proximity. A. caraya is inferred to have diverged from the A. guariba/A. belzebul clade approximately 3.83 million years ago (MYA), with the later pair diverging approximately 1.55 MYA. These dates are much more recent than previous dates based on molecular-clock methods. In addition, analyses of new sequences from the Atlantic Coastal Forest species A. guariba indicate the presence of two distinct haplogroups corresponding to northern and southern populations with both haplogroups occurring in sympatry within Sao Paulo state. The time of divergence of these two haplogroups is estimated to be 1.2 MYA and so follows quite closely after the divergence of A. guariba and A. belzebul. These more recent dates point to the importance of Pleistocene environmental events as important factors in the diversification of A. belzebul and A. guariba. We discuss the diversification of the three Alouatta species in the context of recent models of climatic change and with regard to recent molecular phylogeographic analyses of other animal groups distributed in Brazil.



Similar content being viewed by others
References
Avise JC (2009) Phylogeography: retrospect and prospect. J Biogeogr 36:3–15
Bates JM, Hackett SJ, Cracraft J (1998) Area-relationships in the Neotropical lowlands: a hypotheses based on raw distribution of passerine birds. J Biogeogr 25:783–793
Behling H (1999) Late quaternary vegetational and climatic changes in Brazil. Rev Paleobot Palynol 99:143–156
Behling H (2002) South and southeast Brazilian grasslands during Late Quaternary times: a synthesis. Palaeogeogr Palaeoclimatol Palaeoecol 177:19–27
Behling H, Lichte M (1997) Evidence of dry and cold climatic conditions at glacial times in tropical southeastern Brazil. Quat Res 48:348–358
Bigarella JJ, Andrade-Lima D, Riehs PJ (1975) Consideracões a respeito das mudanças paleoambientais na distribuição de algumas espécies vegetais e animais no Brasil. An Acad Bras Cienc 47:411–464
Bonvicino CR, Lemos B, Seuánez HN (2001) Molecular phylogenetics of howler monkeys (Alouatta, Platyrrhini). A comparison with karyotypic data. Chromosoma 110:241–246
Burgess R, Yang Z (2008) Estimation of hominoid ancestral population sizes under bayesian coalescent models incorporating mutation rate variation and sequencing errors. Mol Biol Evol 25:1979–1994
Bush MB, Gosling WD, Colinvaux PA (2007) Climate change in the lowlands of the Amazon basin. In: Bush MB, Flenley JR (eds) Tropical rainforest responses to climatic change. Springer-Praxis, Berlin, pp 55–76
Cabanne GS, Santos FR, Miyaki CY (2007) Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biol J Linn Soc 91:73–84
Cortés-Ortiz L, Bermingham E, Rico C, Rodríguez-Luna E, Sampaio I, García-Ruiz M (2003) Molecular systematics and biogeography of the neotropical monkey genus, Alouatta. Mol Phylogenet Evol 26:64–81
Costa LP (2003) The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. J Biogeog 30:71–86
Crockett CM (1998) Conservation biology of the genus Alouatta. Int J Primatol 19:549–578
Crockett CM, Eisenberg JF (1987) Howlers: variations in group size and demography. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 54–68
de Oliveira EH (2001) Filogenia da subfamília Atelinae (Primates: Platyrrhini): analises comparativas por pintura cromossômica multicor. Doctoral thesis, Setor de Ciencias Biológicas, Universidad Federal do Paraná, Curitiba
de Oliveira EH, de Lima MMC, Sbalqueiro IJ (1995) Chromosomal variation in Alouatta fusca. Neotrop Primates 3:181–182
de Oliveira PE, Franca-Barreto AM, Suguio K (1999) Late Pleistocene/Holocene climatic and vegetational history of the Brazilian Caatinga: the fossil dunes of the middle Sao Francisco River. Palaeogeogr Palaeoclimatol Palaeoecol 152:319–337
de Oliveira EH, Neusser M, Figueiredo WB, Nagamachi C, Pieczarka JC, Sbalqueiro IJ, Wienberg J, Muller S (2002) The phylogeny of howler monkeys (Alouatta, Platyrrhini): reconstruction by multicolor cross-species chromosome painting. Chrom Res 10:669–683
Ditchfield AD (2000) The comparative phylogeography of Neotropical mammals: patterns of intraespecific mitochondrial DNA variation among bats contrasted to nonvolant small mammals. Mol Ecol 9:1307–1318
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214–221
Edwards SV, Beerli P (2000) Perspective: gene divergence, population divergence and the variance in coalescence time in phylogeographic studies. Evolution 54:1839–1854
Felsenstein J (1985) Confidence limits on phylogeny: using the bootstrap. Evolution 39:783–791
Fu Y-X (1997) Statistical methods, tests of neutrality against population growth, hitchhiking and background selection. Genetics 147:915–925
Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Groves CP (1998) Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol 9:585–598
Grazziotin FG, Monzel M, Echeverrigarauy S, Bonatto S (2006) Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic Forest. Mol Ecol 15:3969–3982
Groves CP (2001) Primate taxonomy. Smithsonian Institution Press, Washington, DC
Harris EE, Gifalli-Iughetti C, Braga ZH, Koiffman CP (2005) Cytochrome b sequences show subdivision between populations of the brown howler monkey (Alouatta guariba) from Rio de Janeiro and Santa Catarina, Brazil. Neotrop Primates 13:16–21
Hasegawa M, Kishino H, Iano T (1985) Dating of the human–ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 21:160–174
Hey J, Nielsen R (2004) Multilocus methods for estimating populations sizes, migration rates and divergence times, with application to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167:747–760
Hey J, Nielsen R (2007) Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. PNAS 104:2785–2790
Hill WCO (1962) Primates comparative anatomy and taxonomy. V. Cebidae. Part. B. Edinburgh University Press, Edinburgh
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HM (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Kimura M (1968) Evolutionary rate at the molecular level. Nature 217:624–626
Kinzey WG (1982) Distribution of primates and forest refuges. In: Prance GT (ed) Biological diversification in the tropics. Columbia University Press, New York, pp 455–482
Kinzey WG (1997) New world primates: ecology. evolution, and behavior (Foundations of Human Behavior Series). Aldine de Gruyter, New York
Ledru M-P (1993) Late quaternary environmental and climatic changes in central Brazil. Quat Res 39:90–98
Ledru M-P, Braga PIS, Soubiés F, Fournier M, Martin L, Suguio K, Turcq B (1996) The last 50,000 years in the Neotropics (Southern Brazil): evolution of vegetation and climate. Palaeogeogr Palaeoclimatol Palaeoecol 123:239–257
Lessa EP, Cook JA, Patton JL (2003) Genetic footprints of demographic expansion in North America, but not Amazonia, during the late quaternary. PNAS 100:10331–10334
Lichte M, Behling H (1999) Dry and cold climatic conditions in the formation of the present landscape in Southeastern Brazil: an interdisciplinary approach to a controversial topic. Zeitschrift fuer Geomorphologie 43:341–358
Lynch JD (1979) The amphibians of the lowland tropical forests. In: Duellman WE (ed) The South American Herpetofauna: its origin, evolution and dispersal. University of Kansas Museum of Natural History, Lawrence, pp 189–216
Martins FM, Meyer D, Ditchfield AD, Morgante JM (2007) Mitochondrial DNA phylogeography reveals marked population structure in the common vampire bat, Desmodus rotundus (Phyllostomidae). J Zool Syst Evol Res 45:372–378
Martins FM, Templeton AR, Pavan ACO, Kohlbach BC, Morgante JM (2009) Phylogeography of the common vampire bat (Desmodus rotundus): marked population structure, Neotropical Pleistocene vicariance and incongruence between nuclear and mtDNA markers. BMC Evol Biol 9:294
Mayle FE, Beerling DJ, Gosling WD, Bush MB (2004) Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the last glacial maximum. Phil Trans R Soc B 359:499–514
Meireles CM, Czelusniak J, Schneider MP, Muniz JAPC, Brigido MC, Ferreira HS, Goodman M (1999) Phylogenetic relationships among Brazilian howler monkeys, genus Alouatta (Platyrrhini, Atelidae), based on γ1 -globin pseudogene sequences. Gen Mol Biol 22:337–344
Moritz C, Hoskin CJ, Mackenzie JB, Phillips BL, Tonione M, Silva N, Vanderwal J, Williams SE, Graham CH (2009) Identification and dynamics of a cryptic suture zone in tropical rainforest. Proc R Soc B 276:1235–1244
Nascimento FF, Bonvicino CR, da Silva FCD, Schneider MPC, Seuánez HN (2005) Cytochrome b polymorphisms and population structure of two species of Alouatta (Primates). Cytogenet Genome Res 108:106–111
Nascimento FF, Bonvicino CR, de Oliveira MM, Schneider MP, Seuánez HN (2007a) Population genetic studies of Alouatta belzebul from the Amazonian and Atlantic Forests. Am J Primatol 70:423–431
Nascimento FF, Bonvicino CR, Seuánez HN (2007b) Population genetic studies of Alouatta caraya (Alouattinae, Primates): inferences on geographic distribution and ecology. Am J Primatol 69:1093–1104
Nordborg M (2001) Coalescent theory. In: Balding D, Cannings C, Bishop M (eds) Handbook of statistical genetics. Wiley, Sussex, pp 843–877
Oliveira-Filho AT, Ratter JA (1995) A study of the origin of central Brazilian forests by the analysis of plant species distribution patterns. Edinb J Bot 52:141–194
Pamilo P, Nei M (1988) The relationships between gene trees and species trees. Mol Biol Evol 5:568–583
Pinto-da-Rocha R, Bragagnolo C, da Silva MB (2005) Faunistic similarity and historical biogeography of the harvestmen of southern and southeastern Atlantic rainforest of Brazil. J Arach 33:290–299
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 19:817–818
Rambaut A (2000) Se Al. http://evolve.zoo.ox.ac.uk/software/Se-Al/
Rannala B, Yang Z (2003) Bayes estimation of species divergence times and ancestral population sizes using DNA sequences from multiple loci. Genetics 164:1645–1656
Rogers A, Harpending HC (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:522–569
Rylands AB, Spironelo WR, Tornisielo VL, Sa RL, de Kierulff MCM, Santos IB (1988) Primates of the Rio Jequitinhonha Valley, Minas Gerais, Brazil. Primate Conserv 9:100–109
Rylands AB, Fonseca GAB, da Leite YLR, Mittermeier RAM (1996) Primates of the Atlantic forest: origin, distributions, endemism, and communities. In: Norconk M, Rosenberger A, Garber PA (eds) Adaptive radiations of Neotropical primates. Plenum Press, New York, pp 21–51
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver. 2000: a software for population genetics data analysis. Switzerland: Genetics and Biometry Laboratory, University of Geneva
Schneider H, Canavez FC, Sampaio I, Moreira MAM, Tagliaro CH, Seuánez HN (2001) Can molecular data place each Neotropical monkey in its own branch? Chromosoma 109:515–523
Steiper ME, Ruvolo M (2003) New World monkey phylogeny based on X-linked G6PD DNA sequences. Mol Phylogenet Evol 27:121–130
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Takahata N, Nei M (1985) Gene genealogy and variance of interpopulational nucleotide sequences. Genetics 110:325–344
Takahata N, Satta Y (1997) Evolution of the primate lineage leading to modern humans: phylogenetic and demographic inferences from DNA sequences. PNAS 94:4811–4815
Tchaicka L, Eizirik E, Oliveira TG, Candido JF Jr, Freitas TRO (2007) Phylogeography and population history of the crab-eating fox (Cerdocyon thous). Mol Ecol 16:819–838
Vanzolini PE (1988) Distributional patterns of South American lizards. In: Vanzolini PE, Hayer W (eds) Proceedings of a workshop on Neotropical distribution patterns: 12–16 January 1987. Academia Brasileira de Ciências, Rio de Janeiro, pp 317–342
Villalobos F, Valerio AA, Retana AP (2004) A phylogeny of howler monkeys (Cebidae: Alouatta) based on mitochondrial, chromosomal, and morphological data. Rev Biol Trop 52:665–677
Vivo M (1997) Mammalian evidence of historical ecological change in the Caatinga semiarid vegetation of northeastern Brazil. J Comp Biol 2:65–73
Wakeley J (2008) Coalescent theory: an introduction. Roberts and Publishers, Greenwood Village
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion (dissertation). The University of Texas, Austin. Available from: http://www.molecularevolution.org/si/software/garli/
Acknowledgments
The authors would like to thank Rodrigo S. dos Santos for critical reviews. Thanks to Marcus V. Domingues, Leon Franciatto, Paulo Noffs, and Jose Grau for the figures. The authors wish to thank Drs Zelinda Hirano Braga and Julio César de Souza Júnior of FURB (Universidade Regional de Blumenau) for samples of Alouatta. We also would like to thank two anonymous reviewers and an anonymous associate editor for detailed and helpful comments. Part of this work was carried out by using the resources of the Computational Biology Service Unit from Cornell University which is partially funded by Microsoft Corporation. This work became possible through financial aid from CAPES PRODOC grant number 0072/087 (to FMM), from CEPED-FAPESP (to CPK), and CUNY RF Awards 67468-00-36 and 68625-0037 (to EEH). Sampling from live animals was carried out in accordance with Brazilian animal care regulations and laws, and also complied with ethical standards for the treatment of animals in the guidelines of the Primate Society of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
de Mello Martins, F., Gifalli-Iughetti, C., Koiffman, C.P. et al. Coalescent analysis of mtDNA indicates Pleistocene divergence among three species of howler monkey (Alouatta spp.) and population subdivision within the Atlantic Coastal Forest species, A. guariba . Primates 52, 77–87 (2011). https://doi.org/10.1007/s10329-010-0226-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-010-0226-2