Abstract
Uncertainty exists as to how to best prevent root caries development. The aim of the present study was to compare sodium fluoride (NaF), chlorhexidine (CHX) and silver diamine fluoride (SDF) varnishes (V) and rinses (R) regarding their caries preventive effect in an artificial caries biofilm model. 140 bovine root dentin samples were cut, polished and embedded. Samples were allocated to seven treatment groups (n = 20/group): Four varnishes (applied once prior biofilm challenge): 38% SDF (SDFV), 35% CHX-varnish (CHXV), 22,600 ppm NaF-varnish (NaFV), placebo-varnish (PV); two rinses (applied once daily during biofilm challenge): 500 ppm NaF solution (NaFR), 0.1% CHX solution (CHXR); one untreated group. Caries was induced in a multi-station, continuous-culture Lactobacillus rhamnosus GG (LGG) biofilm model. Bacteria were inoculated 1 × daily, while 2% sucrose was supplied 8 ×/day followed by artificial saliva for 10 min. After 12 days, mineral loss (ΔZ) was measured in the effect area and adjacent to the varnished areas. Bacterial counts were assessed on de-Man-Rogosa-Sharpe agar. ΔZ was significantly lower in the NaFR group compared with all other groups. Varnishes did not significantly prevent mineral loss in adjacent areas. None of the agents had a significant antimicrobial effect on LGG. Regular fluoride rinses showed highest root caries-preventive effect.
Similar content being viewed by others
Introduction
Given the significant improvements in the prevention of oral diseases, namely caries and periodontitis, in most industrialized countries, even older individuals retain some or most of their natural teeth until high age. During aging, physiological or pathological gingiva recession often occurs, with root surfaces being exposed [1, 2]. These surfaces are at high risk for caries development, as root dentin bears a higher critical pH for mineral loss than enamel [3]. Moreover, elderly populations suffer from hyposalivation more often than younger age groups, while their oral hygiene capacity is increasingly limited, especially when they are burdened with physical or psychological impairment. For those elderly under nursing care, oral health is especially poor and the number of untreated carious teeth high [4,5,6], as adequate oral hygiene can often not be provided by nursing staff [7, 8]. As a consequence, root caries prevalence is high in the elderly, as has been shown for older populations in Germany [9], Britain [10] or the US [11]. Given the growing proportion of elderly in most rich countries, it can be expected that interventions for prevention and management of root caries which are both easy to apply and highly effective will become increasingly important [12].
Clinical trials have investigated a variety of caries-preventive agents for root caries prevention [13,14,15,16,17], most of which showed some kind of beneficial effect. However, due to considerable variations in study parameters, a comparison of the results from these trials is difficult [13, 16]. Moreover, clinical evaluation of caries-preventive agents in high caries-risk populations is often combined with additional oral healthcare [17]. Although design of clinical studies without additional healthcare provision might more closely reflect the true situation of institutionalized elders, conduction of such trials are unethical. To assess the effect of caries preventive agents for patients with high caries-risk without any additional oral healthcare, in vitro studies are needed.
Most caries-preventive agents have been investigated in vitro for their remineralizing or antibacterial activity [18,19,20,21,22,23,24]. However, comparisons are usually made within the same substance class (e.g., remineralizing agents like fluorides against each other or placebo, or antimicrobials like chlorhexidine against each other or placebo). Moreover, different vehicles of administration (e.g., rinses or varnishes) are only sparsely tested against each other, with rinses repeatedly supplying a low-concentrated agent, and varnishes releasing this agent slowly from a highly concentrated reservoir (which additionally covers and potentially protects the surface). Given the heterogeneity of experimental designs, it is difficult to conclude as to which root caries preventive agent is most promising for clinical testing or application.
Therefore, the aim of the present study was to compare different commonly used caries-preventive agents, namely sodium fluoride (NaF), chlorhexidine (CHX) and silver diamine fluoride (SDF) varnishes (V) and rinses (R), regarding their caries-preventive effect on root caries in a continuous-culture biofilm model. Our primary null-hypothesis was that the biofilm-induced mineral loss would not significantly differ between these preventive regimens. Moreover, we hypothesized that none of the different agents would significantly inhibit bacterial colonization on root surfaces in vitro.
Materials and methods
Study design
The influence of different caries-preventive agents on integrated mineral loss (ΔZ) of biofilm-induced dentin carious lesions and bacterial counts was analyzed. For this purpose, 140 bovine root dentin specimens were allocated to seven groups (n = 20 per group): four groups were treated with different varnishes [SDFV, NaFV, CHXV, Placebo-varnish (PV)] once prior biofilm challenge, two groups were treated with different rinses (NaFR, CHXR) once daily during biofilm challenge, and one group received no treatment (control). Name, manufacturer and active ingredients for the different groups are shown in Table 1. Biofilms were grown on specimen surfaces for 12 days. Afterwards, samples were prepared for micro-radiographic analysis of ΔZ and enumeration of bacterial counts of grown biofilm.
Bacterial culture
Lactbacillus rhamnosus GG (LGG, DSM 20021, DSZM, Braunschweig, Germany) was cultured for 48 h (37 °C) on de-Man-Rogosa-Sharpe (MRS) agar (MRS, Difco, Franklin Lakes, USA). Cultures were inoculated into 100 ml of MRS medium with 1% sucrose (MRS-S) and grown similarly for 24 h.
Specimen preparation
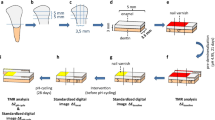
From the roots of 35 bovine incisors of the second dentition, 140 dentin specimens (varnish groups: 2 × 3 × 7 mm, rinse groups and control: 2 × 3 × 4 mm) were prepared (Band Saw EXAKT 300 CL, EXAKT Apparatebau, Norderstedt, Germany), ground flat (LaboPol 25, Struers, Ballerup, Denmark/Willich, Germany) and polished (abrasive paper SiC, P 1000–4000, Buehler, Düsseldorf, Germany). In the varnish group, a notch was created on specimen surface (0.2 mm width, Band Saw EXAKT 300 CL) to define two areas (each 3 × 3 mm). Twenty specimens of each group were transferred into a silicone mould and embedded within acrylic resin (Technovit 4071, Heraeus Kulzer, Hanau, Germany), leaving an uncovered dentin surface of 3 × 6 mm (varnish groups) or 3 × 3 mm (rinse and control groups), respectively (Fig. 1). To remove the smear layer, citric acid (1%) was applied onto the dentin surfaces for 5 min and then thoroughly rinsed with aqua dest. An area of (1 × 3 mm) was covered with nail varnish (Long Lasting Nail Colour, Rival de Loop, Berlin, Germany) to serve as a reference after demineralization. The resulting seven carrier bars were sterilized (121 °C, 2.1 bar, 20 min, Tuttnauer 3870 ELV, Biomedis, Gießen, Germany). For the varnish groups, varnishes were applied with sterile application tips (roundtip applicator regular, Henry Schein, Melville, USA) on the 3 × 3 mm area. To simulate the removal of the varnishes from dentin surfaces by mastication or oral hygiene, the varnished area was brushed with a sterile toothbrush for 30 s followed by rinsing with sterile aqua dest at a temperature of 37 °C for 3 h. On the resulting specimens, the varnishes were not detectable by visual inspection.
Specimen design. Specimens were prepared from the roots of bovine incisors (geometry: varnish groups: 2 × 3 × 7 mm, rinse groups and control: 2 × 3 × 4 mm). A reference area of 1 × 3 mm was covered with nail varnish. For the varnish groups, NaF, CHX or placebo varnish was applied on an area of 3 × 3 mm and removed after 5 min
Artificial mouth setting
The specimens were transferred to a computer-controlled-, multi-station continuous-culture biofilm model [25] with seven different chambers (one carrier bar per chamber) at 100% humidity and 37 °C (Venticell 404 incubator, MMM Medcenter, Planegg, Germany). Supply of nutrition, saliva, bacteria and rinses (in the rinse groups only) was provided by two peristaltic multi-channel pumps (MS/CA, ISMATEC, Wertheim, Germany) to simulate oral conditions. The model had been validated for studies on dentin caries prevention before and found to yield reliable and transferrable results [26, 27]. Prior to the biofilm challenge, a pellicle was formed, by applying 1 ml of sterile-filtrated natural saliva on each carrier bar for 2 h. The natural saliva was obtained from two individuals with low caries risk with informed consent under a reviewed and ethics-approved protocol.
The sequences of daily media provision for the varnish and rinse groups during biofilm challenge is shown in Fig. 2. To simulate adhesion from planktonic LGG in saliva, carrier bars of all groups were inoculated with 5 ml cultures of LGG (approximately 7 × 106 CFU/ml) for 15 min. Groups then were provided with pulses of sterile MRS solution with 1% sucrose (MRS-S) (1 ml/min for 15 min). Ten minutes after each sucrose pulse, modified defined mucin medium (DMM) [28] was provided at 1 ml/min for 15 min followed by a waiting period of 1 h 40 min. The latter sequence (MRS-S–DMM–waiting period) was repeated at total of eight times daily. For the rinse groups, carrier bars were additionally provided with 8.5 ml 500 ppm NaF or 1% CHX solution immediately after the last daily sucrose/DMM sequence, followed by 15 min DMM (1 ml/min) 10 min after the rinse pulse. Overall, cultivation was performed for 12 days, with an overnight resting period of 6 h, 45 min (varnish groups) or, respectively, 6 h, 28 min (rinse groups) per day.
Biofilm analysis
After conclusion of the simulation period, biofilms were isolated from the surface areas of each specimen with a sterile scalpel. For the varnish groups, two biofilms per specimen (one biofilm from the varnished area, one biofilm from the area adjacent to the varnished area) were collected, as varnishes might have an effect not only on varnished but also adjacent surfaces. For all other groups, one biofilm per specimen was collected. Biofilms were inoculated in 1 ml of NaCl (0.9%), vortex mixed, and plated on MRS agar in various dilutions (105–107). Agar plates were cultured at 37 °C and 5% CO2 (CO2 Incubator, Heraeus Kulzer). After 48 h colony-forming units per ml (CFU) were enumerated. Bacterial counts for each specimen were logarithmized (log10 CFU).
Transversal micro-radiography (TMR)
Specimens were cut perpendicularly to the surface into thin sections (Band Saw Exakt 300 cl) and ground to a thickness of 100 ± 10 μm (EXAKT Mikroschleifsystem 400 CS, EXAKT; abrasive paper WS flex 18 C, SiC, P 1200–4000, Hermes Schleifmittel, Hamburg, Germany). Before transferring into the X-ray source, specimens were imbibed in 99% ethylene glycol (Sigma-Aldrich, Steinheim, Germany). Microradiographs of the thin sections were obtained by a nickel-filtered copper X-ray source (X-ray tube PW2213/20, Panalytical, Kassel, Germany; X-ray generator PW 1730/10, Philips, Eindhoven, The Netherlands) operating at 20 kV and 10 mA with an exposure of 10 s. As silver particles within SDF could interact with TMR, and might be dispersed on the sample during polishing, we controlled for the absence of silver using energy-dispersive radiospectroscopy (EDX) in a scanning electron microscope (SEM) (MaXim Camscan, EO, Dortmund, Germany) both on polished and fractured SDF-treated specimens before and after biofilm challenge. Silver dentin concentrations were found to be < 0.5%.
Films (Fine 71337, Fujifilm, Tokyo, Japan) were developed according to the manufacturer’s recommendation under standardized conditions. A digital image-analysing system (CFW 1312 M, Scion, Frederick, USA) interfaced with a universal microscope (CCD-video camera module XC 77 CE, Sony, Tokyo) and a personal computer (TMR for Windows, Version 2.0.27.2, Inspektor Research, Amsterdam, Netherlands) were used to analyze the lesions with regard to ΔZ (vol% × μm). During the preparation for TMR analysis, 5 of the 140 specimens were lost and could not be further analyzed.
Statistical analysis
Statistical analysis was performed with SPSS 22 (IBM, Armonk, USA). Normal distribution of data and homogeneity of variance were checked using the Shapiro–Wilk and Levene tests, respectively. Differences in ΔZ and bacterial counts between different treatment groups were analyzed using one-way analysis of variance (ANOVA). As variances of data were homogeneously distributed, post hoc Tukey test was used to check for significant differences between groups. The level of significance was set at p < 0.05.
Results
ΔZ was significantly lower in the NaFR group than all other groups (p < 0.05, ANOVA/Tukey) (Fig. 3). The second best protection against demineralization was provided by SDF, NaFV and PV. ΔZ for these groups was significantly lower than control. CHXV and CHXR did not significantly protect the dentin as compared with the control group (p > 0.05). Varnishes did not provide any significant protection in adjacent dentin surfaces (p > 0.05).
None of the intervention groups showed significantly lower bacterial counts than the control group (Table 2), while NaFR showed higher bacterial counts than some alternative intervention (p < 0.05).
Discussion
Given the high prevalence of root caries lesions among older adults, there is great need for effective and applicable root caries-preventive regimens. This study compared the caries-preventive effect of different remineralizing and/or antibacterial agents, supplied as varnishes and rinses. We found significant differences of mineral loss between different groups, and thus reject our primary null-hypothesis. We found none of the interventions to significantly reduce bacterial numbers, and thus accept our secondary hypothesis.
Within the present investigation, daily application of NaFR most effectively protected root dentin against mineral loss. This is in accordance with a recently published review, where daily fluoride supply was more effective than varnishing [14]. The caries preventive effect of daily fluoride rinse on root dentin has also been found in other in vitro studies employing pH cycling [24] or Streptococcus mutans biofilm caries models [20]. A relatively high fluoride concentration (500 ppm) was used as NaF-Rinse in the present investigation, as it is provided in over-the-counter products (e.g., Colgate Duraphat Daily Mouthwash) recommended for high caries-risk patients. It might be that higher fluoride concentrations e.g., by additional use of a fluoridated dentifrice lead to more pronounced effects, as the caries-protective effect of fluoride on root dentin was shown to be dose-dependent [20, 24, 29]. There also is evidence that the daily uptake of high fluoride concentrations is more effective for root caries control than low concentrations [30]. In contrast to its caries-preventive effects, NaFR did not inhibit bacterial growth in our study, which contributes to the doubts with regard to low-concentrated fluoride having antibacterial effects [20]. However, higher fluoride concentrations provided as varnish might reduce the number of oral microorganisms clinically [31] although this effect could not be confirmed for the NaFV group in the present investigation.
Within the specific conditions of our study and investigating effects on LGG only, we could not find any antibacterial effect of CHX. This is in accordance with another in vitro investigation, showing CHX rinses to not affect L. rhamnosus cultivation on root dentin [32]. In contrast, CHX has a strong antibacterial effect on S. mutans and other cariogenic microorganisms [16, 33]. It is doubtful if such specific antibacterial effects are sufficient for root caries prevention [13], as lactobacilli are involved in root caries induction [34], and could well replace other, killed cariogenic bacteria in the biofilm [26]. The clinical impact (reduced, increased or unchanged cariogenicity of the biofilm) cannot be deduced from this study. However, as bacterial counts of all of the tested agents and negative control were within 1-LOG, their antibacterial effects might be of minor relevance for caries control.
A number of studies found SDF to have a strong antibacterial activity on cariogenic bacteria before [18, 21, 22, 35]. We could not confirm such effects for LGG, with SDF not providing any significant antibacterial effect in this study. We, therefore, assume the caries-preventive effect of SDF to be attributed to its remineralizing effect, or a potential protection by “sealing” the surface.
Such seal can be postulated, as we found PV to have a caries-preventive effect on dentin. In this study, PV composition was largely similar to that of active varnishes. This protective effect might be due to a protective mechanical barrier being formed on the dentin or within tubules (despite efforts to thoroughly remove all remnants after application). In contrast, another study found no such protection from a PV, despite it being visually detectable after 3 weeks in situ [36]. Clinically, it is unlikely that varnishes would withstand both mastication and oral hygiene on accessible surfaces, but it could be that such deposits remain for longer in sheltered areas, i.e., areas with biofilm stagnation, at risk of lesion induction [37]. Further studies should investigate this aspect.
In contrast to what one might expect, none of the applied varnishes protected adjacent areas from demineralization. We removed the varnishes after application, as we aimed to simulate the removal of the varnishes from dentin surfaces by mastication or oral hygiene. The amount of remaining fluoride or CHX might not have been high enough to protect adjacent areas. That is in line with an in situ study finding fluoride varnish to only protect the varnished surface. However, the same study found CHX to also protect adjacent dentin areas [36]. This difference to our study can be explained by the specific conditions of our study, with CHX not showing any antibacterial effect on LGG (neither on varnished nor on adjacent surfaces). Our finding is relevant, as it could be that varnishes need to be applied precisely where protection is needed.
This study has several limitations. First, a highly cariogenic single-species biofilm model was used. Observed effects might have been different if cariogenic biofilms involving other bacterial species (as they would occur in vivo) would have been additionally employed. However, such biofilms are more complex, and vary both between individuals and settings [38], which makes fully realistic modeling of such biofilms near impossible in vitro [39]. Moreover, lactobacilli are strongly associated with root caries [38], their use in a single-species biofilm model in the purpose of the present investigation might, therefore, be justified. Evidence suggests that L. rhamnosus (amongst other lactobacilli species) is highly prevalent in root caries lesions in elderly [40]. Second, the frequent nutrition supply and undisturbed biofilm growth with daily provision of LGG might have produced a pronounced cariogenic challenge that might not commonly occur under clinical conditions. The additional effect of daily use of fluoridated dentifrice was not tested within our biofilm model, as it was the aim to investigate the caries-preventive potential of each of the used agents in a setting where regular toothbrushing is not necessarily performed (despite being desirable). While the results of our study, therefore, should not be directly translated to clinical practice, our investigation adds evidence to effect of different caries preventive agents in different formulations on root caries. Other caries-preventive factors like fluoride release from oral fluoride reservoirs such as the mucosa were also not simulated in our model, while their relevance might be limited in highly cariogenic environments.
Third, SDF treatment might distort microradiographic findings, as a superposition of silver particles might influence detected mineral losses. There are several in vitro investigations on the caries-preventive effect of SDF using a number of different outcome measures for demineralization [18, 21, 41]. However, analysis of mineral loss by microradiography is seen as one of the most valid methods [42]. We used EDX analysis of both polished and fractured specimens treated with SDF to investigate the possible silver retention on or within dentin. The detected concentration of silver was minimal, which is in accordance with previous findings [43]. We thus assumed silver particles to not greatly impact on TMR analysis.
Last, bovine roots were used for specimen preparation. As only minor differences exist in the demineralization behavior between human and root dentin [44], it should be justified to translate the findings from the present study onto human root dentin. However, it should be noted that the polished, flat surfaces used in our study deviate from the natural root surface anatomy, which is a necessary caveat to perform reliable micro-radiographic evaluation.
In conclusions and within the limitations of the present study, daily fluoride rinsing was most effective for root caries prevention. SDF, sodium fluoride and placebo varnishes were found second best. In contrast, neither CHX rinses nor varnishes had a caries-protective effect. As none of the interventions reduced bacterial numbers, we assume the found protection against demineralization to stem from remineralizing and/or surface-protective effects.
References
Griffin SO, Griffin PM, Swann JL, Zlobin N. Estimating rates of new root caries in older adults. J Dent Res. 2004;83(8):634–8.
Bernabe E, Sheiham A. Age, period and cohort trends in caries of permanent teeth in four developed countries. Am J Public Health. 2014;104(7):e115–21. https://doi.org/10.2105/AJPH.2014.301869.
Hoppenbrouwers PM, Driessens FC, Borggreven JM. The vulnerability of unexposed human dental roots to demineralization. J Dent Res. 1986;65(7):955–8.
Peltola P, Vehkalahti MM, Wuolijoki-Saaristo K. Oral health and treatment needs of the long-term hospitalised elderly. Gerodontology. 2004;21(2):93–9.
Zenthofer A, Rammelsberg P, Cabrera T, Hassel AJ. Increasing dependency of older people in nursing homes is associated with need for dental treatments. Neuropsychiatr Dis Treat. 2014;10:2285–90. https://doi.org/10.2147/NDT.S71184.
Hiraishi N, Yiu CK, King NM, Tagami J, Tay FR. Antimicrobial efficacy of 3.8% silver diamine fluoride and its effect on root dentin. J Endod. 2010;36(6):1026–9. https://doi.org/10.1016/j.joen.2010.02.029.
Chi DL, Berg JH, Kim AS, Scott J. Northwest practice-based RCiE-bD. Correlates of root caries experience in middle-aged and older adults in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J Am Dent Assoc. 2013;144(5):507–16.
Tan HP, Lo EC. Risk indicators for root caries in institutionalized elders. Commun Dent Oral Epidemiol. 2014;42(5):435–40. https://doi.org/10.1111/cdoe.12104.
Michelis W, Schiffner U. Vierte deutsche mundgesundheitsstudie (DMS IV). Köln: DÄV; 2006.
White D, Pitts N, Steele J, Sadler K, Chadwick B. Disease and related disorders—a report from the adult dental health survey 2009. http://www.ic.nhs.uk2011. Accessed Jan 2016
Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat (data from the national health survey). 2007;11(248):1–92.
Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Commun Dent Oral Epidemiol. 2005;33(2):81–92. https://doi.org/10.1111/j.1600-0528.2004.00219.x.
Wierichs RJ, Meyer-Lueckel H. Systematic review on noninvasive treatment of root caries lesions. J Dent Res. 2015;94(2):261–71. https://doi.org/10.1177/0022034514557330.
Gluzman R, Katz RV, Frey BJ, McGowan R. Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dent. 2013;33(3):133–40. https://doi.org/10.1111/j.1754-4505.2012.00318.x.
Walls AW, Meurman JH. Approaches to caries prevention and therapy in the elderly. Adv Dent Res. 2012;24(2):36–40. https://doi.org/10.1177/0022034512453590.
Slot DE, Vaandrager NC, Van Loveren C, Van Palenstein Helderman WH, Van der Weijden GA. The effect of chlorhexidine varnish on root caries: a systematic review. Caries Res. 2011;45(2):162–73. https://doi.org/10.1159/000327374.
Tan HP, Lo EC, Dyson JE, Luo Y, Corbet EF. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89(10):1086–90. https://doi.org/10.1177/0022034510375825.
Mei ML, Chu CH, Low KH, Che CM, Lo EC. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18(6):e824–31.
Hamama HH, Yiu CK, Burrow MF. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust Dent J. 2015;60(1):80–7. https://doi.org/10.1111/adj.12276.
Fernandez CE, Tenuta LM, Cury JA. Validation of a cariogenic biofilm model to evaluate the effect of fluoride on enamel and root dentine demineralization. PLoS ONE. 2016;11(1):e0146478. https://doi.org/10.1371/journal.pone.0146478.
Chu CH, Mei L, Seneviratne CJ, Lo EC. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22(1):2–10. https://doi.org/10.1111/j.1365-263X.2011.01149.x.
Mei ML, Li QL, Chu CH, Lo EC, Samaranayake LP. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4. https://doi.org/10.1186/1476-0711-12-4.
Mei ML, Chu CH, Lo EC, Samaranayake LP. Preventing root caries development under oral biofilm challenge in an artificial mouth. Med Oral Patol Oral Cir Bucal. 2013;18(4):e557–63.
Garcia-Godoy F, Flaitz C, Hicks J. Role of fluoridated dentifrices in root caries formation in vitro. Am J Dent. 2014;27(1):23–8.
Sissons CH, Cutress TW, Hoffman MP, Wakefield JS. A multi-station dental plaque microcosm (artificial mouth) for the study of plaque growth, metabolism, pH, and mineralization. J Dent Res. 1991;70(11):1409–16.
Schwendicke F, Dorfer C, Kneist S, Meyer-Lueckel H, Paris S. Cariogenic effects of probiotic Lactobacillus rhamnosus GG in a dental biofilm model. Caries Res. 2014;48(3):186–92. https://doi.org/10.1159/000355907.
Schwendicke F, Eggers K, Meyer-Lueckel H, Dorfer C, Kovalev A, Gorb S, et al. In vitro Induction of residual caries lesions in dentin: comparative mineral loss and nano-hardness analysis. Caries Res. 2015;49(3):259–65. https://doi.org/10.1159/000371897.
Wong L, Sissons C. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol. 2001;46(6):477–86.
Heijnsbroek M, Paraskevas S, Van der Weijden GA. Fluoride interventions for root caries: a review. Oral Health Prev Dent. 2007;5(2):145–52.
Tavss EA, Mellberg JR, Joziak M, Gambogi RJ, Fisher SW. Relationship between dentifrice fluoride concentration and clinical caries reduction. Am J Dent. 2003;16(6):369–74.
Baygin O, Tuzuner T, Kusgoz A, Senel AC, Tanriver M, Arslan I. Antibacterial effects of fluoride varnish compared with chlorhexidine plus fluoride in disabled children. Oral Health Prev Dent. 2014;12(4):373–82. https://doi.org/10.3290/j.ohpd.a32129.
Zheng CY, Wang ZH. Effects of chlorhexidine, listerine and fluoride listerine mouthrinses on four putative root-caries pathogens in the biofilm. Chin J Dent Res. 2011;14(2):135–40.
Autio-Gold J. The role of chlorhexidine in caries prevention. Oper Dent. 2008;33(6):710–6. https://doi.org/10.2341/08-3.
Papas AS, Vollmer WM, Gullion CM, Bader J, Laws R, Fellows J, et al. Efficacy of chlorhexidine varnish for the prevention of adult caries: a randomized trial. J Dent Res. 2012;91(2):150–5. https://doi.org/10.1177/0022034511424154.
Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40(2):155–61.
Zaura-Arite E, ten Cate JM. Effects of fluoride- and chlorhexidine-containing varnishes on plaque composition and on demineralization of dentinal grooves in situ. Eur J Oral Sci. 2000;108(2):154–61.
Al Dehailan L, Martinez-Mier EA, Lippert F. The effect of fluoride varnishes on caries lesions: an in vitro investigation. Clin Oral Investig. 2015. https://doi.org/10.1007/s00784-015-1648-4.
Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ. Bacterial profiles of root caries in elderly patients. J Clin Microbiol. 2008;46(6):2015–21. https://doi.org/10.1128/JCM.02411-07.
Tang G, Yip HK, Cutress TW, Samaranayake LP. Artificial mouth model systems and their contribution to caries research: a review. J Dent. 2003;31(3):161–71.
Vogel GL. Oral fluoride reservoirs and the prevention of dental caries. Monogr Oral Sci. 2011;22:146–57. https://doi.org/10.1159/000325166.
Mei ML, Ito L, Cao Y, Li QL, Lo EC, Chu CH. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent. 2013;41(9):809–17. https://doi.org/10.1016/j.jdent.2013.06.009.
Arends J, Ruben JL, Inaba D. Major topics in quantitative microradiography of enamel and dentin: R parameter, mineral distribution visualization, and hyper-remineralization. Adv Dent Res. 1997;11(4):403–14.
Willershausen I, Schulte D, Azaripour A, Weyer V, Briseno B, Willershausen B. Penetration potential of a silver diamine fluoride solution on dentin surfaces. An ex vivo study. Clin Lab. 2015;61(11):1695–701.
Lippert F, Churchley D, Lynch RJ. Effect of lesion baseline severity and mineral distribution on remineralization and progression of human and bovine dentin caries lesions. Caries Res. 2015;49(5):467–76. https://doi.org/10.1159/000431039.
Acknowledgements
Silver diamine fluoride (Riva Star™) was supported by SDI Germany GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Göstemeyer, G., Kohls, A., Paris, S. et al. Root caries prevention via sodium fluoride, chlorhexidine and silver diamine fluoride in vitro. Odontology 106, 274–281 (2018). https://doi.org/10.1007/s10266-018-0341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0341-x