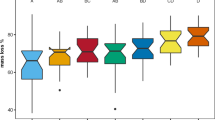
Abstract
Climate and initial litter quality are the major factors influencing decomposition rates on large scales. We established a comprehensive database of terrestrial leaf litter decomposition, including 785 datasets, to examine the relationship between climate and litter quality and evaluate the factors controlling decomposition on a global scale, the arid and semi-arid (AS) zone, the humid middle and humid low (HL) latitude zones. Initial litter nitrogen (N) and phosphorus (P) concentration only increased with mean annual temperature (MAT) in the AS zone and decreased with mean annual precipitation (MAP) in the HL zone. Compared with nutrient content, MAT imposed less effect on initial litter lignin content than MAP. MAT were the most important decomposition driving factors on a global scale as well as in different climatic zones. MAP only significantly affected decomposition constants in AS zone. Although litter quality parameters also showed significant influence on decomposition, their importance was less than the climatic factors. Besides, different litter quality parameters exerted significant influence on decomposition in different climatic zones. Our results emphasized that climate consistently exerted important effects on decomposition constants across different climatic zones.



Similar content being viewed by others
References
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biol 14:2636–2660
Adair EC, Hobbie SE, Hobbie RK (2010) Single-pool exponential decomposition models: potential pitfalls in their use in ecological studies. Ecology 91:1225–1236
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Alvarez-Clare S, Mack M (2011) Influence of precipitation on soil and foliar nutrients across nine Costa Rican forests. Biotropica 43:433–441
Anterola AM, Lewis NG (2002) Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry 61:221–294
Austin AT (2011) Has water limited our imagination for aridland biogeochemistry? Trends Ecol Evol 26:229–235
Austin AT, Sala OE (2002) Carbon and nitrogen dynamics across a natural gradient of precipitation in Patagonia, Argentina. J Veg Sci 13:351–360
Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 113:519–529
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Bakker MA, Carreño-Rocabado G, Poorter L (2011) Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct Ecol 25:473–483
Beier C, Emmett B, Peñuelas J, Schmidt IK, Tietema A, Estiarte M, Gundersen P, Llorens L, Riis-Nielsen L, Sowerby A (2008) Carbon and nitrogen cycles in European ecosystems respond differently to global warming. Sci Total Environ 407:692–697
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bontti EE, Decant JP, Munson SM, Gathany MA, Przeszlowska A, Haddix ML, Owens S, Burke IC, Parton WJ, Harmon ME (2009) Litter decomposition in grasslands of central North America (US great plains). Global Change Biol 15:1356–1363
Botzan TM, Marino MA, Necula AI (1998) Modified de Martonne aridity index: application to the Napa Basin, California. Phys Geogr 19:55–70
Brandt LA, King JY, Milchunas DG (2007) Effects of ultraviolet radiation on litter decomposition depends on precipitation and litter chemistry in a short grass steppe ecosystem. Global Change Biol 13:2193–2205
Brandt L, Bohnet C, King J (2009) Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J Geophys Rese 114:1–13
Chadwick OA, Olson LG, Hendricks DM, Kelly EF, Gavenda RT (1993) Quantifying climatic effects on mineral weathering and neoformation in Hawai’i. In: Transactions of the 15th International Congress of Soil Science, vol 8. Acapulco, Mexico, pp 94–105
Cheng XL, Luo YQ, Su B, Zhou XH, Niu SL, Sherry R, Weng ES, Zhang QF (2010) Experimental warming and clipping altered litter carbon and nitrogen dynamics in a tall grass prairie. Agriculture, Ecosyst Environ 138:206–213
Cleveland CC, Liptzin D (2007) C: N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci USA 103:1031–10321
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Cookson WR, Osman M, Marschner P, Abaye DA, Clark I, Murphy DV, Stockdale EA, Watson C (2007) Controls on soil nitrogen cycling and microbial community composition across land use and incubation temperature. Soil Biol Biochem 39:744–756
Cornelissen JHC, Van Bodegom PM, Aerts R, Callaghan TV, Van Logtestijn RSP, Alatalo J, Stuart Chapin F, Gerdol R, Gudmundsson J, Gwynn-Jones D (2007) Global negative feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10:619–627
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Global Change Biol 15:1339–1355
De Martonne E (1926) Areism and aridity index. Comptes Rendus Hebdomadairesdes Seancesde L’Academiedes Sciences 182:1395–1398
De Santo AV, Berg B, Rutigliano FA, Alfani A, Floretto A (1993) Factors regulating early-stage decomposition of needle litters in five different coniferous forests. Soil Biol Biochem 25:1423–1433
Del Grosso SJ, Parton WJ, Mosier AR, Holland EA, Pendall E, Schimel DS, Ojima DS (2005) Modeling soil CO2 emissions from ecosystems. Biogeochemistry 73:71–91
Diehl P, Mazzarino MJ, Funes F, Fontenla S, Gobbi M, Ferrari J (2003) Nutrient conservation strategies in native Andean-Patagonian forests. J Veg Sci 14:63–70
Emmett BA, Beier C, Estiarte M, Tietema A, Kristensen HL, Williams D, Penuelas J, Schmidt I, Sowerby A (2004) The response of soil processes to climate change: results from manipulation studies of shrublands across an environmental gradient. Ecosystems 7:625–637
Ewing SA, Michalski G, Thiemens M, Quinn RC, Macalady JL, Kohl S, Wankel SD, Kendall C, McKay CP, Amundson R (2007) Rainfall limit of the N cycle on Earth. Global Biogeochem Cycles 21:GB3009
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Fortunel C, Garnier E, Joffre R, Kazakou E, Quested H, Grigulis K, Lavorel S, Ansquer P, Castro H, Cruz P (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 90:598–611
Freschet GT, Weedon JT, Aerts R, van Hal JR, Cornelissen JHC (2012) Interspecific differences in wood decay rates: insights from a new short-term method to study long-term wood decomposition. J Ecol 100:161–170
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053
Gavazov KS (2010) Dynamics of alpine plant litter decomposition in a changing climate. Plant Soil 337:19–32
Hamadi Z, Steinberger Y, Kutiel P, Lavee H, Barness G (2000) Decomposition of Avenasterilis litter under arid conditions. J Arid Environ 46:281–293
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS (2009) Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Global Change Biol 15:1320–1338
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–2644
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hoorens B, Stroetenga M, Aerts R (2010) Litter mixture interactions at the level of plant functional types are additive. Ecosystems 13:90–98
Kang H, Xin Z, Berg B, Burgess PJ, Liu Q, Liu Z, Li Z, Liu C (2010) Global pattern of leaf litter nitrogen and phosphorus in woody plants. Ann Forest Sci 67:811
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litter fall and decomposition in a tropical forest. Ecol Lett 11:35–43
Kimura M, Yamamoto YY, Seki M, Sakurai T, Sato M, Abe T, Yoshida S, Manabe K, Shinozaki K, Matsui M (2003) Identification of Arabidopsis Genes Regulated by High Light-Stress Using cDNA Microarray. Photoch Photobio 77:226–233
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792
Koch M, Schopmeyer S, Kyhn-Hansen C, Madden C (2007) Synergistic effects of high temperature and sulfide on tropical seagrass. J Exp Mar Biol Ecol 341:91–101
Koricheva J, Larsson S, Haukioja E, Keinanen M (1998) Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83:212–226
Kurokawa H, Nakashizuka T (2008) Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology 89:2645–2656
Kurz-Besson C, Coūteaux MM, Thiéry JM, Berg B, Remacle J (2005) A comparison of litterbag and direct observation methods of Scots pine needle decomposition measurement. Soil Biol Biochem 37:2315–2318
Liu C, Berg B, Kutsch W, Westman CJ, Ilvesniemi H, Shen X, Shen G, Chen X (2006) Leaf litter nitrogen concentration as related to climatic factors in Eurasian forests. Global Ecol Biogeo 15:438–444
Magil AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203:301–311
Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Ovreas L, Reysenbach AL, Smith VH, Staley JT (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112
McGroddy ME, Silver WL, Cosme de Oliveira JR (2004) The effect of phosphorus availability on decomposition in a seasonal lowland Amazonian forest. Ecosystems 7:172–179
Meier CL, Bowman WD (2008) Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc Natl Acad Sci USA 105:19780–19785
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Milchunas D, Lauenroth W (2001) Belowground primary production by carbon isotope decay and long-term root biomass dynamics. Ecosystems 4:139–150
Moretto A, Distel R, Didoné N (2001) Decomposition and nutrient dynamic of leaf litter and roots from palatable and unpalatable grasses in a semi-arid grassland. Applied Soil Ecology 18:31–37
Muldavin EH, Moore DI, Collins SL, Wetherill KR, Lightfoot DC (2008) Aboveground net primary production dynamics in a northern Chihuahuan Deserte cosystem. Oecologia 155:123–132
Niklińska M, Maryański M, Laskowski R (1999) Effect of temperature on humus respiration rate and nitrogen mineralization: implications for global climate change. Biogeochemistry 44:239–257
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Palm CA, Sanchez PA (1991) Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic contents. Soil Biol Biochem 23:83–88
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Pendall E, Bridgham S, Hanson PJ, Hungate B, Kicklighter DW, Johnson DW, Law BE, Luo Y, Megonigal JP, Olsrud M, Ryan MG, Wan S (2004) Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phyto 162:311–322
Powers JS, Montgomery RA, Adair EC, Brearley FQ, DeWalt SJ, Castanho CT, Chave J, Deinert E, Ganzhorn JU, Gilbert ME (2009) Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J Ecol 97:801–811
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Prescott C, Vesterdal L, Preston C, Simard S (2004) Influence of initial chemistry on decomposition of foliar litter in contrasting forest types in British Columbia. Canadian J Res 34:1714–1729
R Development Core Team (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramette A, Tiedje JM (2007) Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Pro Natl Acad Sci USA 104:2761–2766
Reed HE, Martiny JBH (2007) Testing the functional significance of microbial composition in natural communities. FEMS Microbiol Ecol 62:161–170
Reed SC, Cleveland CC, Townsend AR (2007) Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39:585–592
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci UAS 101:11001–11006
Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Rutledge S, Campbell DI, Baldocchi D, Schipper LA (2010) Photodegradation leads to increased carbon dioxide losses from terrestrial organic matter. Global Change Biol 16:3065–3074
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88:1126–1131
Santiago L, Schuur E, Silvera K (2005) Nutrient cycling and plant–soil feedbacks along a precipitation gradient in lowland Panama. J Trop Ecol 21:461–470
Schimel DS, House JI, Hibbard KA, Bousquet P, Ciais P, Peylin P, Braswell BH, Apps MJ, Baker D, Bondeau A (2001) Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 414:169–172
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schuur EAG (2001) The effect of water on decomposition dynamics in mesic to wet Hawaiian montane forests. Ecosystems 4:259–273
Semmartin M, Aguiar MR, Distel RA, Moretto AS, Ghersa CM (2004) Litter quality and nutrient cycling affected by grazing-induced species replacements along a precipitation gradient. Oikos 107:148–160
Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change. Ecol Appl 11:1206–1223
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Šimonovičová M, Huttová J, Mistrík I, Široká B, Tamás L (2004) Peroxidase mediated hydrogen peroxide production in barley roots grown under salt stress conditions. Plant Growth Regul 44:267–275
Šnajdr J, Baldrian P (2007) Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotuso streatus and Trametesversi color. Folia Microbiol 52:498–502
Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Re Biol 78:23–56
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculums characteristics. Funct Ecol 23:627–636
Taylor BR, Parkinson D, Parsons WF (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Throop HL, Archer SR (2007) Inter relationships among shrub encroachment, land management, and litter decomposition in a semi desert grassland. Ecol App 17:1809–1823
Toms JD, Lesperance ML (2003) Piecewise regression: a tool for identifying ecological thresholds. Ecology 84:2034–2041
Townsend A, Cleveland C, Houlton B, Alden C, White J (2011) Multi-element regulation of the tropical forest carbon cycle. Frontiers Ecol Environ 9:9–17
Turkey HB (1970) The leaching of substances from plants. Ann Rev Plant Physiol 21:305–324
Vanderbilt K, White C, Hopkins O, Craig J (2008) Aboveground decomposition in arid environments: results of a long-term study in central New Mexico. J Arid Environ 72:696–709
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220
Walther G-R, Post E, Convey P, Menzel A, Parmesanll C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
White MA, Asner GP, Nemani RR, Privette JL, Running SW (2000) Measuring fractional cover and leaf area index in arid ecosystems: digital camera, radiation transmittance, and laser altimetry methods. Remote Sens Environ 74:45–57
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642
Wieder WR, Cleveland CC, Townsend AR (2008) Tropical tree species composition affects the oxidation of dissolved organic matter from litter. Biogeochemistry 88:127–138
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Wood TE, Cavaleri MA, Reed SC (2012) Tropical forest carbon balance in a warmer world: a critical review spanning microbial-to ecosystem-scale processes. Biol Rev 87:912–927
Yuan Z, Chen HYH (2009a) Global trends in senesced-leaf nitrogen and phosphorus. Global Ecol Biogeogr 18:532–542
Yuan Z, Chen HYH (2009b) Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Global Ecol Biogeogr 18:11–18
Yuan ZY, Li LH (2007) Soil water status influences plant nitrogen use: a case study. Plant Soil 301:303–313
Zak DR, Blackwood CB, Waldrop MP (2006) A molecular dawn for biogeochemistry. Trends Ecol Evol 21:288–295
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Acknowledgments
This research was supported by Projects of National Natural Science Foundation of China (31222011, 31270363 and 31321061), National Basic Research Program of China (2013CB956303). University Construction Projects from Central Authorities in Beijing and Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Nanjing Institute of Soil Science, Chinese Academy of Science (Y412201439). We also want to thank Dr. Xin Jing, Dr. Yonghui Wang and the two anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, W. Control of climate and litter quality on leaf litter decomposition in different climatic zones. J Plant Res 128, 791–802 (2015). https://doi.org/10.1007/s10265-015-0743-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0743-6