Abstract
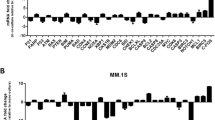
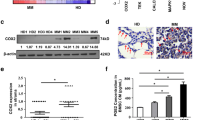
Bone marrow stromal cells (BMSCs) up-regulate B cell-activating factor (BAFF) in multiple myeloma. Increasing experimental evidence has shown that microRNAs play a causal role in hematology tumorigenesis. In this study, we characterized the role of miR-202 in regulating the expression of BAFF in BMSCs. It was found that expressions of BAFF mRNA and protein were increased in BMSCs treated with miR-202 inhibitor. The growth rate of miR-202 mimics transfection cells was significantly lower than that of non-transfected cells. The expression of Bcl-2 protein was down-regulated, and Bax protein was up-regulated after miR-202 mimics transfection. Over-expression of miR-202 in BMSCs rendered MM cells more sensitive to bortezomib. More significantly, the regulatory effect of miR-202 could inhibit the activation of NF-κB pathway in BMSCs. These results suggest that miR-202 functions as a modulator that can negatively regulate BAFF by inhibiting MM cell survival, growth, and adhesion in the bone marrow microenvironment.






Similar content being viewed by others
Abbreviations
- miRNA:
-
microRNA
- BMSCs:
-
Bone marrow stromal cells
- MM:
-
Multiple myeloma
- BAFF:
-
B cell-activating factor
- Bort:
-
Bortezomib
- Thal:
-
Thalidomide
- Dex:
-
Dexamethasone
- TNF:
-
Tumor necrosis factors
- PBMCs:
-
Peripheral blood mononuclear cells
- CAM-DR:
-
Cell adhesion-mediated drug resistance
- ICAM-1:
-
Intercellular adhesion molecule-1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- IL-6:
-
Interleukin 6
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Cömert M, Güneş AE, Sahin F, et al. Quality of life and supportive care in multiple myeloma. Turk J Haematol. 2013;30:234–46.
Andrews SW, Kabrah S, May JE, et al. Multiple myeloma: the bone marrow microenvironment and its relation to treatment. Br J Biomed Sci. 2013;70:110–20.
Terpos E, Christoulas D. Effects of proteasome inhibitors on bone cancer. Bonekey Rep. 2013;2:395.
Moreaux Jérôme, Legouffe Eric, Jourdan Eric. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57.
Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24.
Fragioudaki M, Boula A, Tsirakis G, et al. B cell-activating factor: its clinical significance in multiple myeloma patients. Ann Hematol. 2012;91:1413–8.
Fragioudaki M, Tsirakis G, Pappa CA, et al. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk Res. 2012;36:1004–8.
Shen X, Zhu W, Zhang X, et al. A role of both NF-κB pathways in expression and transcription regulation of BAFF-R gene in multiple myeloma cells. Mol Cell Biochem. 2011;357:21–30.
Xu G, Shen XJ, Pu J, et al. BLyS expression and JNK activation may form a feedback loop to promote survival and proliferation of multiple myeloma cells. Cytokine. 2012;60:505–13.
Tai YT, Li XF, Breitkreutz I, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–82.
Zheng Y, Cai Z, Wang S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–8.
Fuchs O. Targeting of NF-kappaB signaling pathway, other signaling pathways and epigenetics in therapy of multiple myeloma. Cardiovasc Hematol Disord: Drug Targets. 2013;13:16–34.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66.
Kong YW, Ferland-McCollough D, Jackson TJ, et al. MicroRNAs in cancer management. Lancet Oncol. 2012;13:e249–58.
Corsini LR, Bronte G, Terrasi M, et al. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012;16(Suppl 2):S103–9.
Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA. 2008;105:12885–90.
Corthals SL, Sun SM, Kuiper R, et al. MicroRNA signatures characterize multiple myeloma patients. Leukemia. 2011;25:1784–9.
Hao M, Zhang L, An G, et al. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leuk Lymphoma. 2011;52:1787–94.
Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74.
Chi J, Ballabio E, Chen XH, et al. MicroRNA expression in multiple myeloma is associated with genetic subtype, isotype and survival. Biol Direct. 2011;6:23.
Hao M, Zhang L, An G, et al. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leuk Lymphoma. 2011;52:1787–94.
Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–61.
Schrauder MG, Strick R, Schulz-Wendtland R, et al. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS ONE. 2012;7:e29770.
Nymark P, Guled M, Borze I, et al. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos-and histology-related changes in lung cancer. Genes Chromosomes Cancer. 2011;50:585–97.
Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–95.
Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2004;7:585–98.
Li ZW, Chen H, Campbell RA, et al. NF-kappaB in the pathogenesis and treatment of multiple myeloma. Curr Opin Hematol. 2008;15:391–9.
Acknowledgments
Grant support: the National Natural Science Foundation of China (81301498; 81271920); Jiangsu Provincial Program for Medical Innovation Teams and Leading Talents (LJ201133); Scientific Research Subject of Jiangsu Province Health Department (H201422), and the six major human resources project of Jiangsu Province (20012-WS-119), Translational Medicine Project of Affiliated Hospital of Nantong University (TDF-zh201407).
Conflict of interest
The authors have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, X., Guo, Y., Yu, J. et al. miRNA-202 in bone marrow stromal cells affects the growth and adhesion of multiple myeloma cells by regulating B cell-activating factor. Clin Exp Med 16, 307–316 (2016). https://doi.org/10.1007/s10238-015-0355-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0355-4