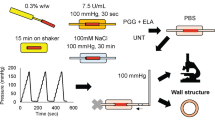
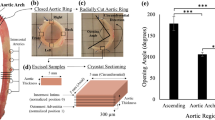
Abstract
Elastic and collagen fibers are well known to be the major load-bearing extracellular matrix (ECM) components of the arterial wall. Studies of the structural components and mechanics of arterial ECM generally focus on elastin and collagen fibers, and glycosaminoglycans (GAGs) are often neglected. Although GAGs represent only a small component of the vessel wall ECM, they are considerably important because of their diverse functionality and their role in pathological processes. The goal of this study was to study the mechanical and structural contributions of GAGs to the arterial wall. Biaxial tensile testing was paired with multiphoton microscopic imaging of elastic and collagen fibers in order to establish the structure–function relationships of porcine thoracic aorta before and after enzymatic GAG removal. Removal of GAGs results in an earlier transition point of the nonlinear stress–strain curves \((p<0.05)\). However, stiffness was not significantly different after GAG removal treatment, indicating earlier but not absolute stiffening. Multiphoton microscopy showed that when GAGs are removed, the adventitial collagen fibers are straighter, and both elastin and collagen fibers are recruited at lower levels of strain, in agreement with the mechanical change. The amount of stress relaxation also decreased in GAG-depleted arteries \((p<0.05)\). These findings suggest that the interaction between GAGs and other ECM constituents plays an important role in the mechanics of the arterial wall, and GAGs should be considered in addition to elastic and collagen fibers when studying arterial function.










Similar content being viewed by others
References
Aikawa J, Munakata H, Isemura M et al (1984) Comparison of Glycosaminoglycans from Thoracic Aortas of several mammals. Tohoku J Exp Med 143:107–112
Ateshian GA, Rajan V, Chahine NO et al (2009) Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J Biomech Eng 131:61003. doi:10.1115/1.3118773
Azeloglu EU, Albro MB, Thimmappa VA et al (2008) Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol 294:H1197–H1205. doi:10.1152/ajpheart.01027.2007
Baker AB, Chatzizisis YS, Beigel R et al (2010) Regulation of heparanase expression in coronary artery disease in diabetic, hyperlipidemic swine. Atherosclerosis 213:436–442. doi:10.1016/j.atherosclerosis.2010.09.003
Baker AB, Groothuis A, Jonas M et al (2009) Heparanase alters arterial structure, mechanics, and repair following endovascular stenting in mice. Circ Res 104:380–387. doi:10.1161/CIRCRESAHA.108.180695
Bautista CA, Park HJ, Mazur CM et al (2016) Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PloS One 11:e0158976. doi:10.1371/journal.pone.0158976
Beenakker J-WM, Ashcroft BA, Lindeman JHN, Oosterkamp TH (2012) Mechanical properties of the extracellular matrix of the Aorta studied by enzymatic treatments. Biophys J 102:1731–1737. doi:10.1016/j.bpj.2012.03.041
Bellini C, Ferruzzi J, Roccabianca S et al (2014) A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng 42:488–502. doi:10.1007/s10439-013-0928-x
Borghi A, New SEP, Chester AH et al (2013) Time-dependent mechanical properties of aortic valve cusps: effect of glycosaminoglycan depletion. Acta Biomater 9:4645–4652. doi:10.1016/j.actbio.2012.09.001
Burton AC (1954) Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev 34:619–642
Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G (1998) Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis 139:205–222. doi:10.1016/S0021-9150(98)00107-5
Cavalcante FSA, Ito S, Brewer K et al (2005) Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98:672–679. doi:10.1152/japplphysiol.00619.2004
Chow M-J, Mondonedo JR, Johnson VM, Zhang Y (2013) Progressive structural and biomechanical changes in elastin degraded aorta. Biomech Model Mechanobiol 12:361–372. doi:10.1007/s10237-012-0404-9
Chow M-J, Turcotte R, Lin CP, Zhang Y (2014) Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J 106:2684–2692. doi:10.1016/j.bpj.2014.05.014
Chow M-J, Zou Y, He H et al (2011) Obstruction-induced pulmonary vascular remodeling. J Biomech Eng 133:111009–111009. doi:10.1115/1.4005301
Couchman JR, Pataki CA (2012) An introduction to proteoglycans and their localization. J Histochem Cytochem 60:885–897. doi:10.1369/0022155412464638
Dingemans KP, Teeling P, Lagendijk JH, Becker AE (2000) Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec 258:1–14. doi:10.1002/(SICI)1097-0185(20000101)258:1<1:AID-AR1>3.0.CO;2-7
Eckert CE, Fan R, Mikulis B et al (2013) On the biomechanical role of glycosaminoglycans in the aortic heart valve leaflet. Acta Biomater 9:4653–4660. doi:10.1016/j.actbio.2012.09.031
Evanko SP, Tammi MI, Tammi RH, Wight TN (2007) Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev 59:1351–1365. doi:10.1016/j.addr.2007.08.008
Fessel G, Snedeker JG (2009) Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol 28:503–510. doi:10.1016/j.matbio.2009.08.002
Fry JL, Shiraishi Y, Turcotte R et al (2015) Vascular smooth muscle sirtuin-1 protects against aortic dissection during angiotensin II-induced hypertension. J Am Heart Assoc 4:e002384. doi:10.1161/JAHA.115.002384
Grashow JS, Yoganathan AP, Sacks MS (2006) Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng 34:315–325. doi:10.1007/s10439-005-9027-y
Halper J (2014) Proteoglycans and Diseases of Soft Tissues. In: Halper J (ed) Progress in heritable soft connective tissue diseases. Springer, The Netherlands, pp 49–58
Hayes AJ, Lord MS, Smith SM et al (2011) Colocalization in vivo and association in vitro of perlecan and elastin. Histochem Cell Biol 136:437–454. doi:10.1007/s00418-011-0854-7
Humphrey JD (2013) Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-beta. J Vasc Res 50:1–10. doi:10.1159/000342436
Jamal RA, Roughley PJ, Ludwig MS (2001) Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cell Mol Physiol 280:L306–L315
Lai WM, Hou JS, Mow VC (1991) A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng 113:245–258
Legerlotz K, Riley GP, Screen HRC (2013) GAG depletion increases the stress-relaxation response of tendon fascicles, but does not influence recovery. Acta Biomater 9:6860–6866. doi:10.1016/j.actbio.2013.02.028
Lillie MA, Gosline JM (2006) Tensile residual strains on the rlastic lamellae along the porcine thoracic aorta. J Vasc Res 43:587–601. doi:10.1159/000096112
Lovekamp JJ, Simionescu DT, Mercuri JJ et al (2006) Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials 27:1507–1518. doi:10.1016/j.biomaterials.2005.08.003
Maroudas AI (1976) Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 260:808–809
Mow VC, Kuei SC, Lai WM, Armstrong CG (1980) Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng 102:73–84. doi:10.1115/1.3138202
Murienne BJ, Jefferys JL, Quigley HA, Nguyen TD (2015) The effects of glycosaminoglycan degradation on the mechanical behavior of the posterior porcine sclera. Acta Biomater 12:195–206. doi:10.1016/j.actbio.2014.10.033
Provenzano P, Lakes R, Keenan T, Vanderby R (2001) Nonlinear ligament viscoelasticity. Ann Biomed Eng 29:908–914. doi:10.1114/1.1408926
Reinboth B, Hanssen E, Cleary EG, Gibson MA (2002) Molecular interactions of biglycan and decorin with elastic fiber components. J Biol Chem 277:3950–3957. doi:10.1074/jbc.M109540200
Rigozzi S, Müller R, Stemmer A, Snedeker JG (2013) Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J Biomech 46:813–818. doi:10.1016/j.jbiomech.2012.11.017
Robinson PS, Lin TW, Reynolds PR et al (2004) Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng 126:252–257. doi:10.1115/1.1695570
Roccabianca S, Ateshian GA, Humphrey JD (2014a) Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol 13:13–25. doi:10.1007/s10237-013-0482-3
Roccabianca S, Bellini C, Humphrey JD (2014) Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface 11:20140397–20140397. doi:10.1098/rsif.2014.0397
Sacks MS, Chuong CJ (1998) Orthotropic mechanical properties of chemically treated bovine pericardium. Ann Biomed Eng 26:892–902. doi:10.1114/1.135
Scott JE (2003) Elasticity in extracellular matrix “shape modules” of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol 553:335–343. doi:10.1113/jphysiol.2003.050179
Takahashi A, Majumdar A, Parameswaran H et al (2014) Proteoglycans maintain lung stability in an elastase-treated mouse model of emphysema. Am J Respir Cell Mol Biol 51:26–33. doi:10.1165/rcmb.2013-0179OC
Tan X, Sanderson MJ (2014) Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br J Pharmacol 171:646–662. doi:10.1111/bph.12460
Tovar AM, Cesar DC, Leta GC, Mourão PA (1998) Age-related changes in populations of aortic glycosaminoglycans: species with low affinity for plasma low-density lipoproteins, and not species with high affinity, are preferentially affected. Arterioscler Thromb Vasc Biol 18:604–614
Turcotte R, Mattson JM, Wu JW et al (2016) Molecular order of arterial collagen using circular polarization second-harmonic generation imaging. Biophys J 110:530–533. doi:10.1016/j.bpj.2015.12.030
Wagenseil JE, Mecham RP (2009) Vascular extracellular matrix and arterial mechanics. Physiol Rev 89:957–989. doi:10.1152/physrev.00041.2008
Wang F, Kim MS, Puthanveetil P et al (2009) Endothelial heparanase secretion after acute hypoinsulinemia is regulated by glucose and fatty acid. Am J Physiol Heart Circ Physiol 296:H1108–1116. doi:10.1152/ajpheart.01312.2008
Wight TN (1980) Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb 5:1–39
Zeinali-Davarani S, Chow M-J, Turcotte R, Zhang Y (2013) Characterization of biaxial mechanical behavior of porcine aorta under gradual elastin degradation. Ann Biomed Eng 41:1528–1538. doi:10.1007/s10439-012-0733-y
Zeller PJ, Skalak TC (1998) Contribution of individual structural components in determining the zero-stress state in small arteries. J Vasc Res 35:8–17. doi:10.1159/000025560
Zhu W, Iatridis JC, Hlibczuk V et al (1996) Determination of collagen-proteoglycan interactions in vitro. J Biomech 29:773–783. doi:10.1016/0021-9290(95)00136-0
Zou Y, Zhang Y (2010) The orthotropic viscoelastic behavior of aortic elastin. Biomech Model Mechanobiol 10:613–625. doi:10.1007/s10237-010-0260-4
Acknowledgments
This work was supported, in part, by a Grant from National Science Foundation (CMMI 1463390) and a predoctoral training grant from National Institutes of Health (2T32HL007969).
Conflict of interest
The authors confirm that there are no conflicts of interest associated with this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattson, J.M., Turcotte, R. & Zhang, Y. Glycosaminoglycans contribute to extracellular matrix fiber recruitment and arterial wall mechanics. Biomech Model Mechanobiol 16, 213–225 (2017). https://doi.org/10.1007/s10237-016-0811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0811-4