Abstract
Ross operation, i.e., the use of autologous pulmonary artery to replace diseased aortic valve, has been recently at the center of a vivid debate regarding its unjust underuse in the surgical practice. Keystone of the procedure regards the use of an autologous biologically available graft which would preserve the anticoagulative and tissue homeostatic functions normally exerted by the native leaflets and would harmoniously integrate in the vascular system, allowing for progressive somatic growth of aortic structures. With this respect, recently, some of the authors have successfully pioneered a large animal model of transposition of pulmonary artery in systemic pressure load in order to reproduce the clinical scenario in which this procedure might be applied and allow for the development and testing of different devices or techniques to improve the pulmonary autograft (PA) performance, by testing a bioresorbable mesh for PA reinforcement. In the present work, to support and supplement the in vivo animal experimentation, a mathematical model is developed in order to simulate the biomechanical changes in pulmonary artery subjected to systemic pressure load and reinforced with a combination of resorbable and auxetic synthetic materials. The positive biological effects on vessel wall remodeling, the regional somatic growth phenomena and prevention of dilatative degeneration have been analyzed. The theoretical outcomes show that a virtuous biomechanical cooperation between biological and synthetic materials takes place, stress-shielding guiding the physiological arterialization of vessel walls, consequently determining the overall success of the autograft system.








Similar content being viewed by others
Notes
From a rigorous mechanical point of view, the term “residual stress” —although widely used in the literature—is often incorrectly adopted: In fact, in the absence of applied loads, before to ideally (and suitably) cut a grown material, the stress kindled within the tissue as a result of the inhomogeneous growth is “self-equilibrated” rather than “residual” (“remaining”), while—after cutting an inhomogeneously grown material—a “stress-free” (or “stress-relaxed”) deformed configuration due to the full or partial stress relieved through the cut can be recovered rather than a “residual stress”. The adjective “residual” is hence erroneous in the first case and ambiguous in the second, because it suggests that one should find residual stresses after cutting a (grown) material, while on the contrary a “stress-free” (or “stress-relaxed”) deformed configuration characterized by “residual (inelastic) strain” is actually found. This is the reason for which, in the present work, the terms “residual strain”, “self-equilibrated (residual) stress” and/or “stress-free deformed state” have been preferred.
There is thus a direct effect of the PDS pauperization on the growth, remodeling and in turn on the overall elastic properties of the adventitia, but not vice versa, that is, growth and remodeling do not affect (or perturb) the PDS degradation law.
References
Alastrué V, Peña E, Martínez MÁ, Doblaré M (2007) Assessing the use of the “opening angle method” to enforce residual stresses in patient-specific arteries. Ann Biomed Eng 35(10):1821–1837
Alastrué V, Peña E, Martínez MÁ, Doblaré M (2008) Experimental study and constitutive modelling of the passive mechanical properties of the ovine infrarenal vena cava tissue. J Biomech 41(14):3038–3045
Ambrosi D, Ateshian GA, Arruda EM, Cowin SC, Dumais J, Goriely A, Holzapfel GA, Humphrey JD, Kemkemer R, Kuhl E, Olberding JE, Taber LA, Garikipati K (2011) Perspectives on biological growth and remodeling. J Mech Phys Solids 59(4):863–883
Ambrosi D, Guana F (2005) Stress-modulated growth. Math Mech Solids 12(3):319–342
Ambrosi D, Guillou A (2007) Growth and dissipation in biological tissues. Contin Mech Thermodyn 19(5):245–251
Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL (2005) Electrospinning polydioxanone for biomedical applications. Acta Biomater 1(1):115–123
Cabrera M, Oomens C, Bouten C, Bogers A, Hoerstrup S, Baaijens F (2013) Mechanical analysis of ovine and pediatric pulmonary artery for heart valve stent design. J Biomech 46(12):2075–2081
Centola M, Rainer A, Spadaccio C, De Porcellinis S, Genovese JA, Trombetta M (2010) Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2(1):14102
Chuong CJ, Fung YC (1983) Three-dimensional stress distribution in arteries. J Biomech Eng 105(3):268–274
Coleman BD, Gurtin ME (1967) Thermodynamics with internal state variables. J Chem Phys 47(2):597–613
Cowin SC, Doty SB (2007) Tissue mechanics. Springer, Berlin
Fraldi M, Guarracino F (2011) An improved formulation for the assessment of the capacity load of circular rings and cylindrical shells under external pressure. part 1. analytical derivation. Thin-Walled Struct 49(9):1054–1061
Fraldi M, Carannante F, Nunziante L (2013) Analytical solutions for n-phase functionally graded material cylinders under de saint venant load conditions: Homogenization and effects of poisson ratios on the overall stiffness. Compos B Eng 45(1):1310–1324
Fraldi M, Nunziante L, Gesualdo A, Guarracino F (2010) On the bounding of limit multipliers for combined loading. Proc R Soc A Math Phys Eng Sci 466(2114):493–514
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52(8):1263–1334
Fung Y (1981) Biomechanics: mechanical properties of living tissues. Biomechanics / Y. C. Fung. Springer, Berlin
Hamouda MB, Atti N (2011) Comparison of growth curves of lamb fat tail measurements and their relationship with body weight in babarine sheep. Small Rumin Res 95(2–3):120–127
Holzapfel G, Gasser T, Ogden R (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast Phys Sci solids 61(1–3):1–48
Holzapfel GA, Ogden RW (2006) Mechanics of biological tissue. Springer, Berlin
Holzapfel GA, Ogden RW (2010) Constitutive modelling of arteries. Proc R Soc Lond A Math Phys Eng Sci 466(2118):1551–1597
Horer J, Hanke T, Stierle U, Takkenberg JJM, Bogers AJJC, Hemmer W, Rein JG, Hetzer R, Hubler M, Robinson DR, Sievers HH, Lange R (2009) Neoaortic root diameters and aortic regurgitation in children after the Ross operation. Ann Thorac Surg 88(2):594–600 discussion 600
Humphrey JD (2002) Cardiovasc Solid Mech. Springer, New York
Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, Donaldson MC, Whittemore AD, Conte MS (2004) Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. J Vasc Surg 39(3):547–555
Karkach A (2006) Trajectories and models of individual growth. Demograp Res 15:347–400
Kim S-H, Mun C, Jung Y, Kim S-H, Kim D-I, Kim S (2013) Mechanical properties of compliant double layered poly(l-lactide-co-\(\epsilon \)-caprolactone) vascular graft. Macromol Res 21(8):886–891
Kováčik J (1999) Correlation between young’s modulus and porosity in porous materials. J Mater Sci Lett 18(13):1007–1010
Lu S, Zhang P, Sun X, Gong F, Yang S, Shen L, Huang Z, Wang C (2013) Synthetic ePTFE grafts coated with an anti-CD133 antibody-functionalized heparin/collagen multilayer with rapid in vivo endothelialization properties. ACS Appl Mater Interfaces 5(15):7360–7369
Lubarda VA, Hoger A (2002) On the mechanics of solids with a growing mass. Int J Solids Struct 39(18):4627–4664
Molea G, Schonauer F, Bifulco G, D’Angelo D (2000) Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (polydioxanone, poliglecaprone 25, glycomer 631). Br J Plast Surg 53(2):137–141
Nappi F, Spadaccio C, Castaldo C, Di Meglio F, Nurzynska D, Montagnani S, Chello M, Acar C (2014) Reinforcement of the pulmonary artery autograft with a polyglactin and polydioxanone mesh in the Ross operation: experimental study in growing lamb. J Heart Valve Dis 23(2):145–148
Nappi F, Spadaccio C, Al-Attar N, Acar C (2015a) The ross procedure at the crossroads: lessons from biology: is dr ross’s dream concluded? Int J Cardiol 178:37–39
Nappi F, Spadaccio C, Chello M, Acar C (2015b) The Ross procedure: underuse or under-comprehension? J Thorac Cardiovasc Surg 149(5):1463–1464
Nappi F, Spadaccio C, Fouret P, Hammoudi N, Chachques JC, Chello M, Acar C (2015c) An experimental model of the Ross operation: development of resorbable reinforcements for pulmonary autografts. J Thorac Cardiovasc Surg 149(4):1134–1142
Nuutinen J-P, Clerc C, Reinikainen R, Törmälä P (2003) Mechanical properties and in vitro degradation of bioabsorbable self-expanding braided stents. J Biomater Sci Polym Ed 14(3):255–266
Olsson T, Klarbring A (2008) Residual stresses in soft tissue as a consequence of growth and remodeling: application to an arterial geometry. Eur J Mech A Solids 27(6):959–974
Rechtsman MC, Stillinger FH, Torquato S (2008) Negative poisson’s ratio materials via isotropic Interactions. Phys Rev Lett 101(8):85501
Ren J-S (2013) Growth and residual stresses of arterial walls. J Theor Biol 337:80–88
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467
Sabino MA, González S, Márquez L, Feijoo JL (2000) Study of the hydrolytic degradation of polydioxanone PPDX. Polym Degrad Stab 69(2):209–216
Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ (2001) The mechanical behavior of vascular grafts: a review. J Biomater Appl 15(3):241–278
Sell S, McClure M, Knapp D, Walpoth B, Simpson DG, Bowlin GL (2006) Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomed Mater 1:72–80
Sonoda H, Urayama S-I, Takamizawa K, Nakayama Y, Uyama C, Yasui H, Matsuda T (2002) Compliant design of artificial graft: Compliance determination by new digital x-ray imaging system-based method. J Biomed Mater Res 60(1):191–195
Spadaccio C, Chello M, Trombetta M, Rainer A, Toyoda Y, Genovese JA (2009) Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med 13(3):422–439
Spadaccio C, Rainer A, Centola M, Trombetta M, Chello M, Lusini M, Covino E, Toyoda Y, Genovese JA (2010) Heparin-releasing scaffold for stem cells: a differentiating device for vascular aims. Regen Med 5(4):645–657
Spadaccio C, Montagnani S, Acar C, Nappi F (2015) Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int J Cardiol 190:50–52
Tai NR, Salacinski HJ, Edwards A, Hamilton G, Seifalian A (2000) Compliance properties of conduits used in vascular reconstruction. Br J Surg 87(11):1516–1524
Turing AM (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biolog Sci 237(641):37–72
Vandiver R, Goriely A (2009) Differential growth and residual stress in cylindrical elastic structures. Philos Trans R Soc A Math Phys Eng Sci 367(1902):3607–3630
Veselý J, Horný L, Chlup H, Adámek T, Krajíček M, Žitný R (2015) Constitutive modeling of human saphenous veins at overloading pressures. J Mech Behav Biomed Mater 45:101–108
Wolfram Research I (2015) Mathematica. Wolfram Research, Inc, Champaign
Zhao S, Gu L (2014) Implementation and validation of aortic remodeling in hypertensive rats. J Biomech Eng 136(9):91007
Zilberman M, Eberhart RC (2006) Drug-eluting bioresorbable stents for various applications. Annu Rev Biomed Eng 8(1):153–180
Acknowledgments
The authors would like to thank the anonymous reviewers for their valuable comments and helpful suggestions which have improved the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Details on the in vivo animal model
The experimental model of transposition of the pulmonary trunk as autograft in aortic position has been developed and performed under cardiopulmonary bypass in 20 growing lambs (Nappi et al. 2014, 2015b, c). Technical and anatomical issues imposed reimplantation of the PA in the descending aorta, with the pulmonary trunk being replaced by a homograft from another lamb of the same age and weight (Fig. 3a). The age of the animals at the moment of the implant was 2 months (8–10 weeks), and baseline mean weight was about 21\(\pm \)3 kg, allowing to observe the progression of the autograft diameter during the period of fastest growth. Morphometric and cardiovascular parameters were comparable preoperatively among animals. There was no difference in hemoglobin levels and ventricular function. The group of 20 lambs was divided into two subgroups: a control group (n\(=\)10), subjected to ordinary PA transposition, and a group of 10 animals in which the PA was reinforced with an external synthetic semiresorbable armored scaffold (prosthetic). All animal experiments have been performed in respect of the guidelines for animal care and handling, and the protocol was approved by the institutional animal care committee.
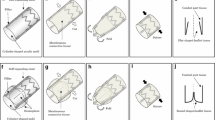
Semiresorbable copolymer scaffold The experimental design of the device consisted of an internal bioresorbable scaffold made with Polydioxanone (PDS), arranged in a frame of hexagonal cells, externally coupled with a nonresorbable layer of e-PTFE, having an auxetic behavior. The mesh structure and arrangement were specifically designed in order to constrain the excessive enlargement of the vascular graft diameter by also carrying wall mechanical stress while accommodating its natural longitudinal growth by embracing the root of the aorta. For this purpose, the unit cells of the PDS and e-PTFE plies have been, respectively, positioned as sketched in Fig. 2.
Surgical Model Lambs were premedicated with ketamine (25mg / kg via intramuscular injection), and anesthesia was guaranteed by the injection of sodium thiopentothal (6–8mg / kg) via the internal jugular. Animal received 100mg of lidocaine intravenously as prophylaxis against rhythm disturbance. After endotracheal intubation, ventilation was provided up to animal awakening and the anesthesia was maintained with inhalation isoflurane (1–2.5\(\%\)). The electrocardiogram was monitored, and chest was prepped and shaved. The heart was approached via left thoracotomy. After opening the pericardium, the right atrium was exposed for cannulation and the trunk of the pulmonary artery was dissected free from its right ventricular origin up to its bifurcation in the pulmonary arteries. The same was done for the descending thoracic aorta, and a region distal to the portion of choice for the PA transposition was cannulated. Approximately 8 cm of the descending thoracic aorta was left for clamp positioning and to perform the anastomosis with the pulmonary artery trunk under optimal conditions. Heparin (3mg/kg) was administered intravenously, and cardiopulmonary bypass was started between right atrium and descending aorta. The cerebral circulation of the animal was guaranteed on a beating heart. A 3-cm tract of pulmonary artery trunk was transposed into the descending aorta with an end-to-end anastomosis in 5-0 prolene. A fresh pulmonary artery homograft, explanted from animals killed on the same day for another experimental study, was inserted to reconstruct the right outflow tract, with a proximal and distal end-to-end anastomosis in 5-0 prolene, as in the Ross operation. Left thoracotomy was closed and aspiration drainage left in place. Before implantation, in the experimental group, the PA has been reinforced with PDS and e-PTFE meshes according to the study design. The resorbable mesh was prepared at the operative table (time 10\(\pm \)2 min). Meshes used in this study were cut into a rectangle measuring 20mm in height matching with the height of autograft and rolled out on a metallic candle and then reassured by a suture to create a cylinder with an internal diameter of 10mm (20mm in height in 10mm diameter directly adherent to the PA). The autograft was then inserted into the fibrillar cylinder and was anastomosed suturing both its margins and those of the prosthetic structure to the pulmonary autograft trunk. The mesh was oriented to allow maximal extensibility in the longitudinal direction and minimal transverse extensibility. All animals survived to the procedure and did not experience surgical complications. A case of PA initial rupture and thrombosis occurred at 6-month follow-up in the control group, without causing animal decease. Procedure did not pose particular technical challenges. At 6 months, the lambs weight was doubled ( 21\(\pm \)3kg at day 0 and 55\(\pm \)10kg at 6 months), suggesting a normal growth process. The animal model was mainly focused on the development of an effective and reproducible model of pulmonary autograft transposition into arterial system with the aim to study the behavior of the autograft and develop suitable strategies to prevent its future dilation, which represents one of the major drawback of this operation.
Appendix B:Thermodynamic forces associated with the growth model
The theoretical model presents a kinematic description of the growth process based on the deformation gradient decomposition into an elastic and a growth tensor, whose components are determined through both the evolution Eq. (2.2) and the introduction of an anisotropy exponent see (2.3). The evolution equation represents a kinematic relation for the body elementary growing volume; in particular, by introducing a growth volumetric source (and sink) term \(r_g\), under the assumptions of no mass fluxes and constant density \(\rho \) (which implies a pure volumetric growth), the mass conservation for the elastically incompressible body (i.e., \(J_e=1\)) can be expressed by:
In order to describe the growth behavior of the experimental animal models and reproduce the effects of the physiological growth on the stresses in vessel walls, a logistic rate has been assumed in the form of (2.35). This hypothesis implies that growth and the other model variables are uncoupled and thermodynamic conjugate forces have to be derived for the dissipative problem at hand. To make this, the constitutive laws involving the Piola-Kirchhoff stress in (2.10) and the remodeling Eq. (2.11) have been here derived from a dissipation principle, by following the approach by Lubarda and Hoger (2002) and Olsson and Klarbring (2008), in this way in turn determining the explicit face of the growth-associated forces. In particular, under the hypothesis of isothermal process, the balance of energy can be written by taking into account a contribution to the growth which represents a metabolic energy supply per unit mass, say \(\varepsilon _g\), and a vector of driving forces \(\mathbf {k}\) responsible of the remodeling-associated microstructural changes. In this way, one finally obtains:
u and \(\mathcal {V}\) being the internal energy per unit current mass and the current volume measure, respectively. Also, \(\mathbf {v}\) is the velocity vector and \(\mathbf {d}=sym(\dot{\mathbf {F}}\mathbf {F}^{-1})\) is the symmetrical velocity gradient, the other quantities \(\varvec{\sigma }\) and \(\dot{\varvec{\gamma }}\) defining the Cauchy stress tensor and the rate of the remodeling parameter vector, as also specified in the main text. By using (4.1), the balance of energy (4.2) reduces to:
The total internal dissipation per unit initial mass can be instead accounted by introducing two thermodynamic forces \(f_g\) and \(\varvec{f}_{\gamma }\), respectively, conjugated to the rates \(r_g\) and \(\dot{\varvec{\gamma }}\): In such a way, the rate of dissipation is written down:
where s is the entropy per unit current mass and \(\theta \) is the absolute temperature. The second law of thermodynamics requires the right side of (4.4) to be nonnegative. By combining the energy Eq. (4.3) and the entropy Eq. (4.4), the free energy per unit volume \(\psi =\rho \left( u-\theta \,s\right) \) can be thus obtained as a function upon the elastic deformation \(\mathbf {F}_e\) and the remodeling parameters \(\varvec{\gamma }\), as also established in (2.9). At the end, it results:
\({\mathcal {V}^0}\) denoting the referential volume. By means of the localization theorem and exploiting the deformation multiplicative decomposition \(\mathbf {F}=\mathbf {F}_e\mathbf {F}_g\), one has:
A direct comparison of the terms at both sides of (4.7) leads to:
from which it follows that the growth-conjugate force is the result of the interplay of metabolic (e.g., biochemical) and mechanical factors, with \(\Sigma =\varvec{\Sigma }:\mathbf {I}\) being the trace of the Eshelby-like stress tensor related to the change in domain variations induced by the volumetric growth \(\varvec{\Sigma }=\mathbf {F}_e^T\partial \psi /\partial \mathbf {F}_e-\psi \mathbf {I}\), as obtained for example by Ambrosi and Guillou 2007 and Olsson and Klarbring 2008. As a consequence, the dissipation inequality, derivable by imposing the second member of (4.4) to be nonnegative, can be split into two independent contributions:
The first one leads to the form of the (2.11) through the assumption \(\dot{\varvec{\gamma }}=c_{\gamma }\left( \mathbf {K}-J_g{\partial \psi }/{\partial \varvec{\gamma }}\right) , c_{\gamma }>0\) and \(\mathbf {K}=J_g\mathbf {k}\) being the referential force which drives the remodeling process. The second inequality is a pressure–volume relationship (vanishing in the pure remodeling case, \(\dot{J}_g = 0\)). Its validity implies that the growth case \(\dot{J}_g>0\) is characterized by the presence of a pressure responsible of the domain expansion and by an adequate amount of metabolic energy, convertible into mass growth, that is assumed to be indefinitely available; vice versa, the resorption case \(\dot{J}_g < 0\) can be associated with the lack of energy supply and to the presence of stresses contracting the volume domain.
Rights and permissions
About this article
Cite this article
Nappi, F., Carotenuto, A.R., Di Vito, D. et al. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech Model Mechanobiol 15, 1141–1157 (2016). https://doi.org/10.1007/s10237-015-0749-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0749-y