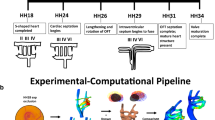
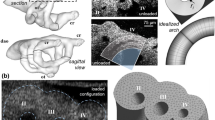
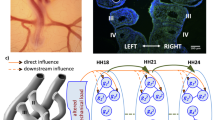
Abstract
In the early embryo, a series of symmetric, paired vessels, the aortic arches, surround the foregut and distribute cardiac output to the growing embryo and fetus. During embryonic development, the arch vessels undergo large-scale asymmetric morphogenesis to form species-specific adult great vessel patterns. These transformations occur within a dynamic biomechanical environment, which can play an important role in the development of normal arch configurations or the aberrant arch morphologies associated with congenital cardiac defects. Arrested migration and rotation of the embryonic outflow tract during late stages of cardiac looping has been shown to produce both outflow tract and several arch abnormalities. Here, we investigate how changes in flow distribution due to a perturbation in the angular orientation of the embryonic outflow tract impact the morphogenesis and growth of the aortic arches. Using a combination of in vivo arch morphometry with fluorescent dye injection and hemodynamics-driven bioengineering optimization-based vascular growth modeling, we demonstrate that outflow tract orientation significantly changes during development and that the associated changes in hemodynamic load can dramatically influence downstream aortic arch patterning. Optimization reveals that balancing energy expenditure with diffusive capacity leads to multiple arch vessel patterns as seen in the embryo, while minimizing energy alone led to the single arch configuration seen in the mature arch of aorta. Our model further shows the critical importance of the orientation of the outflow tract in dictating morphogenesis to the adult single arch and accurately predicts arch IV as the dominant mature arch of aorta. These results support the hypothesis that abnormal positioning of the outflow tract during early cardiac morphogenesis may lead to congenital defects of the great vessels due to altered hemodynamic loading.
Similar content being viewed by others
References
Alford PW, Humphrey JD, Taber LA (2008) Growth and remodeling in a thick-walled artery model: effects of spatial variations in wall constituents. Biomech Model Mechanobiol 7(4): 245–262. doi:10.1007/s10237-007-0101-2
Baba K, Kawamura T, Shibata M, Sohirad M, Kamiya A (1995) Capillary-tissue arrangement in the skeletal muscle optimized for oxygen transport in all mammals. Microvasc Res 49(2): 163–179. doi:10.1006/mvre.1995.1013
Backer CL, Mavroudis C (1997) Surgical approach to vascular rings. Adv Card Surg 9: 29–64
Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME (2006) Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res 98(3): 421–428. doi:10.1161/01.RES.0000202800.85341.6e
Barry A (1951) The aortic arch derivatives in the human adult. Anat Rec (Hoboken) 111(2): 221–238
Bayer IM, Adamson SL, Langille BL (1999) Atrophic remodeling of the artery-cuffed artery. Arter Thromb Vasc Biol 19(6): 1499–1505
Beloussov LV (2008) Mechanically based generative laws of morphogenesis. Phys Biol 5(1): 015009. doi:10.1088/1478-3975/5/1/015009
Beloussov LV, Grabovsky VI (2006) Morphomechanics: goals, basic experiments and models. Int J Dev Biol 50(2–3): 81–92. doi:10.1387/ijdb.052056lb
Bostrom MP, Hutchins GM (1988) Arrested rotation of the outflow tract may explain double-outlet right ventricle. Circulation 77(6): 1258–1265
Brauner CJ, Matey V, Wilson JM, Bernier NJ, Val AL (2004) Transition in organ function during the evolution of air-breathing: insights from Arapaima gigas, an obligate air-breathing teleost from the Amazon. J Exp Biol 207(Pt 9): 1433–1438
Bremer JL (1928) Experiments on the aortic arches in the chick. Anat Rec (Hoboken) 37(3): 225–254
Chen CY, Patrick MJ, Corti P, Kowalski WJ, Roman BL, Pekkan K (2011) Early embryonic great-vessel microcirculation in zebrafish using high-speed confocal μPIV and volume-of-fluid methods. Biorheology (accepted)
Collette Y, Siarry P (2003) Multiobjective optimization: principles and case studies. Springer, Berlin
Congdon ED, Wang HD (1926) The mechanical processes concerned in the formation of the different types of aortic arches in the chick and pig and the divergent early development of their pulmonary arches. Am J Anat 37: 499–520
Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, Roman BL (2011) Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138(8): 1573–1582. doi:10.1242/dev.060467
Culver JC, Dickinson ME (2010) The effects of hemodynamic force on embryonic development. Microcirculation 17(3): 164–178. doi:10.1111/j.1549-8719.2010.00025.x
deAlmeida A, McQuinn T, Sedmera D (2007) Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res 100(9): 1363–1370. doi:10.1161/01.RES.0000266606.88463.cb
de la Cruz MV, Sanchez Gomez C, Arteaga MM, Arguello C (1977) Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123(Pt 3): 661–686
Dor X, Corone P (1985) Migration and torsions of the conotruncus in the chick embryo heart: observational evidence and conclusions drawn from experimental intervention. Heart Vessels 1(4): 195–211
Dur O, Wang Y, Patrick M, Tinney J, Keller B, Pekkan K (2009) Correlation of wall shear stress and pharyngeal arch lumen diameter during early embryonic development in the chick. Paper presented at the BMES annual fall meeting, Pittsburgh, 8 Oct
Dur O, Coskun TS, Coskun OK, Frakes D, Kara LB, Pekkan K (2011) Computer-aided patient-specific coronary artery graft design improvements using CFD coupled shape optimizer. Cardiovasc Eng Technol 2(1): 35–47
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85(1): 97–177. doi:10.1152/physrev.00050.2003
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46): 3583–3602. doi:10.1016/j.cma.2008.09.013
Flynn ME, Pikalow AS, Kimmelman RS, Searls RL (1991) The mechanism of cervical flexure formation in the chick. Anat Embryol (Berl) 184(4): 411–420
Fung YC (1990) Biomechanics: motion, flow, stress, and growth. Springer, New York
Gessner IH (1966) Spectrum of congenital cardiac anomalies produced in chick embryos by mechanical interference with cardiogenesis. Circ Res 18(6): 625–633
Girerd X, London G, Boutouyrie P, Mourad JJ, Safar M, Laurent S (1996) Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27(3 Pt 2): 799–803
Gleason RL, Humphrey JD (2004) A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J Vasc Res 41(4): 352–363. doi:10.1159/000080699
Goor DA, Edwards JE (1973) The spectrum of transposition of the great arteries: with specific reference to developmental anatomy of the conus. Circulation 48(2): 406–415
Groenendijk BC, Hierck BP, Gittenberger-De Groot AC, Poelmann RE (2004) Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn 230(1): 57–68. doi:10.1002/dvdy.20029
Gruionu G, Hoying JB, Pries AR, Secomb TW (2005) Structural remodeling of mouse gracilis artery after chronic alteration in blood supply. Am J Physiol Heart Circ Physiol 288(5): H2047–H2054. doi:10.1152/ajpheart.00496.2004
Hacking WJ, VanBavel E, Spaan JA (1996) Shear stress is not sufficient to control growth of vascular networks: a model study. Am J Physiol 270(1 Pt 2): H364–H375
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1): 49–92
Hanke M (2004) Benchmarking FEMLAB 3.0a: laminar flows in 2D. Report No. 2004:01
Hiruma T, Hirakow R (1995) Formation of the pharyngeal arch arteries in the chick embryo: observations of corrosion casts by scanning electron microscopy. Anat Embryol (Berl) 191(5): 415–423
Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (1999) Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res 41(1): 87–99
Holzapfel GA, Ogden RW (2010) Constitutive modelling of arteries. Proc R Soc Math Phy 466(2118): 1551–1596. doi:10.1098/rspa.2010.0058
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61(1–3): 1–48
Hu N, Clark EB (1989) Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65(6): 1665–1670
Hu N, Christensen DA, Agrawal AK, Beaumont C, Clark EB, Hawkins JA (2009) Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anat Rec (Hoboken) 292(5): 652–660. doi:10.1002/ar.20885
Hudetz AG, Kiani MF (1992) The role of wall shear stress in microvascular network adaptation. Adv Exp Med Biol 316: 31–39
Hughes GM, Morgan M (1973) Structure of fish gills in relation to their respiratory function. Biol Rev 48(3): 419–475
Humphrey JD (1999) Remodeling of a collagenous tissue at fixed lengths. J Biomech Eng 121(6): 591–597
Kamiya A, Togawa T (1980) Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol 239(1): H14–H21
Kamiya A, Takeda S, Shibata M (1987) Optimum capillary number for oxygen delivery to tissue in man. Bull Math Biol 49(3): 351– 361
Kamiya A, Wakayama H, Baba K (1993) Optimality analysis of vascular-tissue system in mammals for oxygen transport. J Theor Biol 162(2): 229–242. doi:10.1006/jtbi.1993.1084
Kardong KV (2009) Vertebrates: comparative anatomy, function, evolution, 5th edn. McGraw-Hill Higher Education, Boston
Kassab GS, Fung YC (1995) The pattern of coronary arteriolar bifurcations and the uniform shear hypothesis. Ann Biomed Eng 23(1): 13–20
Keenan RL, Rodbard S (1973) Competition between collateral vessels. Cardiovasc Res 7(5): 670–675
Khanin MA, Bukharov IB (1994) Optimal structure of the microcirculatory bed. J Theor Biol 169(3): 267–273. doi:10.1006/jtbi.1994.1147
LaBarbera M (1990) Principles of design of fluid transport systems in zoology. Science 249(4972): 992–1000
Langille BL, O’Donnell F (1986) Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231(4736): 405–407
Li D, Robertson AM (2009) A structural multi-mechanism damage model for cerebral arterial tissue. J Biomech Eng 131(10): 101013. doi:10.1115/1.3202559
Liem KF, Bemis WE, Walker WF, Grande L (2001) Functional anatomy of the vertebrates: an evolutionary perspective, 3rd edn. Thomson Brooks/Cole, Belmont
Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF (2002) Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129(21): 5081–5091
Lomonico MP, Bostrom MP, Moore GW, Hutchins GM (1988) Arrested rotation of the outflow tract may explain tetralogy of Fallot and transposition of the great arteries. Pediatr Pathol 8(3): 267–281
Lu X, Zhao JB, Wang GR, Gregersen H, Kassab GS (2001) Remodeling of the zero-stress state of femoral arteries in response to flow overload. Am J Physiol Heart Circ Physiol 280(4): H1547–H1559
Lucitti JL, Tobita K, Keller BB (2005) Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J Exp Biol 208(Pt 10): 1877–1885. doi:10.1242/jeb.01574
Manner J (2000) Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259(3): 248–262. doi:10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K
Manner J, Seidl W, Steding G (1993) Correlation between the embryonic head flexures and cardiac development—an experimental-study in chick-embryos. Anat Embryol 188(3): 269– 285
Manner J, Seidl W, Steding G (1995) Formation of the cervical flexure—an experimental-study on chick-embryos. Acta Anat 152(1): 1–10
Marler RT, Arora JS (2004) Survey of multi-objective optimization methods for engineering. Struct Multidiscip Optim 26(6): 369–395. doi:10.1007/s00158-003-0368-6
Martinsen BJ (2005) Reference guide to the stages of chick heart embryology. Dev Dyn 233(4): 1217–1237. doi:10.1002/dvdy.20468
McDougall SR, Anderson AR, Chaplain MA, Sherratt JA (2002) Mathematical modelling of flow through vascular networks: implications for tumour-induced angiogenesis and chemotherapy strategies. Bull Math Biol 64(4): 673–702. doi:10.1006/bulm.2002.0293
McElhinney DB, Tworetzky W, Lock JE (2010) Current status of fetal cardiac intervention. Circulation 121(10): 1256–1263. doi:10.1161/circulationaha.109.870246
Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS (1993) Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem 4(2): 105–111
Murray CD (1926) The physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proc Natl Acad Sci USA 12(3): 207–214
Patrick MJ, Chen CY, Frakes DH, Dur O, Pekkan K (2011) Cellular-level near-wall unsteadiness of high-hematocrit erythrocyte flow using confocal μPIV. Exp Fluids 50(4): 887–904. doi:10.1007/s00348-010-0943-8
Patten BM (1920) The early embryology of the chick. P. Blakiston’s Son & Co, Philadelphia
Pekkan K, Dur O, Sundareswaran K, Kanter K, Fogel M, Yoganathan A, Undar A (2008) Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J Biomech Eng 130(6): 061012. doi:10.1115/1.2978988
Piiper J (1982) Respiratory gas exchange at lungs, gills and tissues: mechanisms and adjustments. J Exp Biol 100: 5–22
Poelmann RE, Gittenberger-de Groot AC, Hierck BP (2008) The development of the heart and microcirculation: role of shear stress. Med Biol Eng Comput 46(5): 479–484. doi:10.1007/s11517-008-0304-4
Pries AR, Secomb TW, Gaehtgens P (1995) Design principles of vascular beds. Circ Res 77(5): 1017–1023
Pries AR, Secomb TW, Gaehtgens P (1998) Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol 275(2 Pt 2): H349–H360
Pries AR, Reglin B, Secomb TW (2001) Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol 281(3): H1015–H1025
Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D (2003) Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res 93(1): 77–85. doi:10.1161/01.RES.0000079488.91342.B7
Rodbard S (1975) Vascular caliber. Cardiology 60(1): 4–49
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4): 455–467
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4): e18–e209. doi:10.1161/CIR.0b013e3182009701
Rychter Z (1962) Experimental morphology of the aortic arches and the heart loop in chick embryos. Adv Morphog 2: 333–371
Rychter Z, Lemez L (1965) Changes in localization in aortic arches of laminar blood streams of main venous trunks to heart after exclusion of vitelline vessels on second day of incubation. Fed Proc Transl Suppl 24(5): 815–820
Sadler TW, Langman J (2006) Langman’s medical embryology, 10th edn. Lippincott Williams & Wilkins, Philadelphia
Schittowski K (1985) NLQPL: a FORTRAN-subroutine solving constrained nonlinear programming problems. Ann Oper Res 5: 485–500
Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB (1999) Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254(2): 238–252. doi:10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V
Sherman TF (1981) On connecting large vessels to small. The meaning of Murray’s law. J Gen Physiol 78(4): 431–453
Shibeshi SS, Collins WE (2005) The rheology of blood flow in a branched arterial system. Appl Rheol 15(6): 398–405. doi:10.1901/jaba.2005.15-398
Sun S, Wheeler MF, Obeyesekere M, Patrick CW Jr. (2005) A deterministic model of growth factor-induced angiogenesis. Bull Math Biol 67(2): 313–337. doi:10.1016/j.bulm.2004.07.004
Taber LA (1998a) A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng 120(3): 348–354
Taber LA (1998b) An optimization principle for vascular radius including the effects of smooth muscle tone. Biophys J 74(1): 109–114. doi:10.1016/S0006-3495(98)77772-0
Taber LA (2009) Towards a unified theory for morphomechanics. Phil Trans A Math Phys Eng Sci 367(1902): 3555–3583. doi:10.1098/rsta.2009.0100
Taber LA, Eggers DW (1996) Theoretical study of stress-modulated growth in the aorta. J Theor Biol 180(4): 343–357. doi:10.1006/jtbi.1996.0107
Taber LA, Humphrey JD (2001) Stress-modulated growth, residual stress, and vascular heterogeneity. J Biomech Eng 123(6): 528–535
Thompson RP, Abercrombie V, Wong M (1987) Morphogenesis of the truncus arteriosus of the chick embryo heart: movements of autoradiographic tattoos during septation. Anat Rec 218(4):434–440, 394–435. doi:10.1002/ar.1092180411
Tobita K, Garrison JB, Liu LJ, Tinney JP, Keller BB (2005) Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol 283(1): 193–201. doi:10.1002/ar.a.20133
Valentin A, Humphrey JD, Holzapfel GA (2011) A multi-layered computational model of coupled elastin degradation, vasoactive dysfunction, and collagenous stiffening in aortic aging. Ann Biomed Eng 39(7): 2027–2045. doi:10.1007/s10439-011-0287-4
Wagenseil JE (2010) A constrained mixture model for developing mouse aorta. Biomech Model Mechanobiol. doi:10.1007/s10237-010-0265-z
Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, Keller BB, Pekkan K (2009) Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng 37(6): 1069–1081. doi:10.1007/s10439-009-9682-5
Watton PN, Raberger NB, Holzapfel GA, Ventikos Y (2009) Coupling the hemodynamic environment to the evolution of cerebral aneurysms: computational framework and numerical examples. J Biomech Eng 131(10): 101003. doi:10.1115/1.3192141
Yashiro K, Shiratori H, Hamada H (2007) Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450(7167): 285–288. doi:10.1038/nature06254
Yoshigi M, Knott GD, Keller BB (2000) Lumped parameter estimation for the embryonic chick vascular system: a time-domain approach using MLAB. Comput Methods Programs Biomed 63(1): 29–41
Zamir M (1977) Shear forces and blood vessel radii in the cardiovascular system. J Gen Physiol 69(4): 449–461
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The Below is the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Kowalski, W.J., Teslovich, N.C., Dur, O. et al. Computational hemodynamic optimization predicts dominant aortic arch selection is driven by embryonic outflow tract orientation in the chick embryo. Biomech Model Mechanobiol 11, 1057–1073 (2012). https://doi.org/10.1007/s10237-012-0373-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0373-z