Abstract
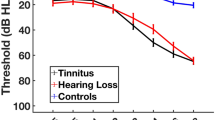
Speech-in-noise (SiN) recognition difficulties are often reported in patients with tinnitus. Although brain structural changes such as reduced gray matter (GM) volume in auditory and cognitive processing regions have been reported in the tinnitus population, it remains unclear how such changes influence speech understanding, such as SiN performance. In this study, pure-tone audiometry and Quick Speech-in-Noise test were conducted on individuals with tinnitus and normal hearing and hearing-matched controls. T1-weighted structural MRI images were obtained from all participants. After preprocessing, GM volumes were compared between tinnitus and control groups using whole-brain and region-of-interest analyses. Further, regression analyses were performed to examine the correlation between regional GM volume and SiN scores in each group. The results showed decreased GM volume in the right inferior frontal gyrus in the tinnitus group relative to the control group. In the tinnitus group, SiN performance showed a negative correlation with GM volume in the left cerebellum (Crus I/II) and the left superior temporal gyrus; no significant correlation between SiN performance and regional GM volume was found in the control group. Even with clinically defined normal hearing and comparable SiN performance relative to controls, tinnitus appears to change the association between SiN recognition and regional GM volume. This change may reflect compensatory mechanisms utilized by individuals with tinnitus who maintain behavioral performance.



Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Tyler RS, Baker LJ (1983) Difficulties experienced by tinnitus sufferers. J Speech Hear Disord 48(2):150–154. https://doi.org/10.1044/jshd.4802.150
Vielsmeier V, Kreuzer PM, Haubner F, Steffens T, Semmler PRO, Kleinjung T, Schlee W, Langguth B, Schecklmann M (2016) Speech comprehension difficulties in chronic tinnitus and its relation to hyperacusis. Front Aging Neurosci 8:293. https://doi.org/10.3389/fnagi.2016.00293
Ivansic D, Guntinas-Lichius O, Müller B, Volk GF, Schneider G, Dobel C (2017) Impairments of speech comprehension in patients with tinnitus-a review. Front Aging Neurosci 9:224. https://doi.org/10.3389/fnagi.2017.00224
Tai Y, Husain FT (2018) Right-ear advantage for speech-in-noise recognition in patients with nonlateralized tinnitus and normal hearing sensitivity. J Assoc Res Otolaryngol 19(2):211–221. https://doi.org/10.1007/s10162-017-0647-3
Tai Y, Husain FT (2020) Association between tinnitus pitch and consonant recognition in noise. Am J Audiol 29(4):916–929. https://doi.org/10.1044/2020_AJA-20-00050
Janse E (2012) A non-auditory measure of interference predicts distraction by competing speech in older adults. Aging Neuropsychol Cogn 19(6):741–758. https://doi.org/10.1080/13825585.2011.652590
Peelle JE (2018) Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear 39(2):204–214. https://doi.org/10.1097/AUD.0000000000000494
Ashburner J, Friston KJ (2000) Voxel-based morphometry - the methods. Neuroimage 11(6):805–821. https://doi.org/10.1006/nimg.2000.0582
Ashburner J, Friston KJ (2001) Why voxel-based morphometry should be used. Neuroimage 14(6):1238–1243. https://doi.org/10.1006/nimg.2001.0961
Kanai R, Rees G (2011) The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci 12:231–242. https://doi.org/10.1038/nrn3000
Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D (2006) Structural brain changes in tinnitus. Cereb Cortex 16(9):1283–1288. https://doi.org/10.1093/cercor/bhj070
Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G (2009) Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46(1):213–218. https://doi.org/10.1016/j.neuroimage.2009.01.069
Allan TW, Besle J, Langers DRM, Davies J, Hall DA, Palmer AR, Adjamian P (2016) Neuroanatomical alterations in tinnitus assessed with magnetic resonance imaging. Front Aging Neurosci 8:221. https://doi.org/10.3389/fnagi.2016.00221
Melcher JR, Knudson IM, Levine RA (2013) Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies ( > 8 kHz ), but not with tinnitus. Hear Res 295:79–86. https://doi.org/10.1016/j.heares.2012.03.013
Boyen K, Langers DRM, de Kleine E, van Dijk P (2013) Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear Res 295:67–78. https://doi.org/10.1016/j.heares.2012.02.010
Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, Horwitz B (2011) Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res 1369:74–88. https://doi.org/10.1016/j.brainres.2010.10.095
Koops EA, de Kleine E, van Dijk P (2020) Gray matter declines with age and hearing loss, but is partially maintained in tinnitus. Sci Rep 10:21801. https://doi.org/10.1038/s41598-020-78571-0
Vanneste S, Van De Heyning P, De Ridder D (2015) Tinnitus: a large VBM-EEG correlational study. PLoS ONE 10(3):e0115122. https://doi.org/10.1371/journal.pone.0115122
Rudner M, Seeto M, Keidser G, Johnson B, Ronnberg J (2019) Poorer speech reception threshold in noise is associated with lower brain volume in auditory and cognitive processing regions. J Speech Lang Hear Res 62(4S):1117–1130. https://doi.org/10.1044/2018_JSLHR-H-ASCC7-18-0142
Wong PCM, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S (2010) Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear 31(4):471–479. https://doi.org/10.1097/AUD.0b013e3181d709c2
Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S (2004) Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am 116(4):2395–2405. https://doi.org/10.1121/1.1784440
American National Standards Institute (2003) Maximum permissible ambient noise levels for audiometric test rooms
Tillman TW, Carhart R (1966) An expanded test for speech discrimination utilizing CNC monosyllabic words: Northwestern University Auditory Test No. 6. Northwestern University Auditory Research Lab, Evanston, IL
American National Standards Institute (2010) Specification for audiometers
Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, Myers PJ, Newman CW, Sandridge S, Turk DC, Folmer RL, Frederick EJ, House JW, Jacobson GP, Kinney SE, Martin WH, Nagler SM, Reich GE, Searchfield G, Sweetow R, Vernon JA (2012) The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 33(2):153–176. https://doi.org/10.1097/AUD.0b013e31822f67c0
Henry JA, Griest S, Thielman E, Mcmillan G, Kaelin C, Carlson KF (2016) Tinnitus Functional Index: development, validation, outcomes research, and clinical application. Hear Res 334:58–64. https://doi.org/10.1016/j.heares.2015.06.004
Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D (2006) Structural brain changes in tinnitus. Cereb Cortex 16(9):1283–1288. https://doi.org/10.1093/cercor/bhj070
Maldjian JA, Laurienti PJ, Burdette JH (2004) Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21(1):450–455. https://doi.org/10.1016/j.neuroimage.2003.09.032
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239. https://doi.org/10.1016/S1053-8119(03)00169-1
Schmidt SA, Zimmerman B, Bido Medina RO, Carpenter-Thompson JR, Husain FT (2018) Changes in gray and white matter in subgroups within the tinnitus population. Brain Res 1679:64–74. https://doi.org/10.1016/j.brainres.2017.11.012
Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA (2009) Speech recognition in younger and older adults: a dependency on low-level auditory cortex. J Neurosci 29(19):6078–6087. https://doi.org/10.1523/JNEUROSCI.0412-09.2009
Peelle JE, Wingfield A (2016) The neural consequences of age-related hearing loss. Trends Neurosci 39(7):486–497. https://doi.org/10.1016/j.tins.2016.05.001
Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC (2007) The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav 1:3. https://doi.org/10.1007/s11682-007-9000-5
Deschamps I, Hasson U, Tremblay P (2016) The structural correlates of statistical information processing during speech perception. PLoS One 11(2):e0149375. https://doi.org/10.1371/journal.pone.0149375
Merabet LB, Pascual-Leone A (2010) Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci 11(1):44–52. https://doi.org/10.1038/nrn2758
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Changes in grey matter induced by training. Nature 427:311–312. https://doi.org/10.1038/427311a
Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P (2010) Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30(35):11670–11677. https://doi.org/10.1523/JNEUROSCI.2567-10.2010
Cardin V, Orfanidou E, Ronnberg J, Capek CM, Rudner M, Woll B (2013) Dissociating cognitive and sensory neural plasticity in human superior temporal cortex. Nat Commun 4:1473. https://doi.org/10.1038/ncomms2463
Lövdén M, Wenger E, Mårtensson J, Lindenberger U, Bäckman L (2013) Structural brain plasticity in adult learning and development. Neurosc Biobehav Rev 37(9):2296–2310. https://doi.org/10.1016/j.neubiorev.2013.02.014
Hickok G, Poeppel D (2000) Towards a functional neuroanatomy of speech perception. Trends Cog Sci 4(4):131–138. https://doi.org/10.1016/S1364-6613(00)01463-7
Kennedy-Higgins D, Devlin JT, Nuttall HE, Adank P (2020) The causal role of left and right superior temporal gyri in speech perception in noise: a transcranial magnetic stimulation study. J Cog Neurosci 32(6):1092–1103. https://doi.org/10.1162/jocn_a_01521
Davis MH, Ford MA, Kherif F, Johnsrude IS (2011) Does semantic context benefit speech understanding through “top-down” processes? Evidence from time-resolved sparse fMRI. J Cog Neurosci 23(12):3914–3932. https://doi.org/10.1162/jocn_a_00084
Evans S, McGettigan C, Agnew ZK, Rosen S, Scott SK (2016) Getting the cocktail party started: Masking effects in speech perception. J Cog Neurosci 28(3):483–500. https://doi.org/10.1162/jocn_a_00913
Narain C, Scott SK, Wise RJS, Rosen S, Leff A, Iversen SD, Matthews PM (2003) Defining a left-lateralized response specific to intelligible speech using fMRI. Cereb Cortex 13(12):1362–1368. https://doi.org/10.1093/cercor/bhg083
Vander Ghinst M, Bourguignon Op, de Beeck M, Wens V, Marty B, Hassid S, Choufani G, Jousmäki V, Hari R, Van Bogaert P, Goldman S, De Tiège X (2016) Left superior temporal gyrus is coupled to attended speech in a cocktail-party auditory scene. J Neurosci 36(5):1596–1606. https://doi.org/10.1523/JNEUROSCI.1730-15.2016
Wong PCM, Uppunda AK, Parrish TB, Dhar S (2008) Cortical mechanisms of speech perception in noise. J Speech Lang Hear Res 51(4):1026–1041. https://doi.org/10.1044/1092-4388(2008/075)
Bauer CA, Kurt W, Sybert LT, Brozoski TJ (2013) The cerebellum as a novel tinnitus generator. Hear Res 295:130–139. https://doi.org/10.1016/j.heares.2012.03.009
Mennink LM, van Dijk JMC, van Dijk P (2020) The cerebellar (para)flocculus: a review on its auditory function and a possible role in tinnitus. Hear Res 398:108081. https://doi.org/10.1016/j.heares.2020.108081
Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, Habas C, Hagura N, Ivry RB, Marien P, Molinari M, Naito E, Nowak DA, Ben Taib NO, Pelisson D, Tesche CD, Tilikete C, Timmann D (2012) Consensus paper: Roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. Cerebellum 11:457–487. https://doi.org/10.1007/s12311-011-0331-9
Guediche S, Holt LL, Laurent P, Lim SJ, Fiez JA (2015) Evidence for cerebellar contributions to adaptive plasticity in speech perception. Cereb Cortex 25(7):1867–1877. https://doi.org/10.1093/cercor/bht428
Mathiak K, Hertrich I, Grodd W, Ackermann H (2002) Cerebellum and speech perception: a functional magnetic resonance imaging study. J Cog Neurosci 14(6):902–912. https://doi.org/10.1162/089892902760191126
Petacchi A, Laird AR, Fox PT, Bower JM (2005) Cerebellum and auditory function: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp 25(1):118–128. https://doi.org/10.1002/hbm.20137
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44(2):489–501. https://doi.org/10.1016/j.neuroimage.2008.08.039
Durisko C, Fiez JA (2010) Functional activation in the cerebellum during working memory and simple speech tasks. Cortex 46(7):896–906. https://doi.org/10.1016/j.cortex.2009.09.009
Yuan P, Raz N (2014) Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev 42:180–192. https://doi.org/10.1016/j.neubiorev.2014.02.005
Bureš Z, Profant O, Svobodová V, Tóthová D, Vencovský V, Syka J (2019) Speech comprehension and its relation to other auditory parameters in elderly patients with tinnitus. Front Aging Neurosci 11:219. https://doi.org/10.3389/FNAGI.2019.00219
Barnea G, Attias J, Gold S, Shahar A (1990) Tinnitus with normal hearing sensitivity: Extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiol 29(1):36–45. https://doi.org/10.3109/00206099009081644
Brännström KJ, Karlsson E, Waechter S, Kastberg T (2018) Extended high-frequency pure tone hearing thresholds and core executive functions. Int J Audiol 57(9):639–645. https://doi.org/10.1080/14992027.2018.1475755
Yoo HB, De Ridder D, Vanneste S (2016) The importance of aging in gray matter changes within tinnitus patients shown in cortical thickness, surface area and volume. Brain Topogr 29(6):885–896. https://doi.org/10.1007/s10548-016-0511-5
Acknowledgements
The authors would like to thank Dr. Anthony Tsao for assisting with audiological data collection and Gibbeum Kim for assisting with data analysis.
Funding
The study was supported by the Department of Defense, award number W81XWH-15-2-0032 (PI: Husain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tai, Y., Shahsavarani, S., Khan, R.A. et al. An Inverse Relationship Between Gray Matter Volume and Speech-in-Noise Performance in Tinnitus Patients with Normal Hearing Sensitivity. JARO 24, 385–395 (2023). https://doi.org/10.1007/s10162-023-00895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-023-00895-1