Abstract
The middle-ear system relies on a balance of mass and stiffness characteristics for transmitting sound from the external environment to the cochlea and auditory neural pathway. Phase is one aspect of sound that, when transmitted and encoded by both ears, contributes to binaural cue sensitivity and spatial hearing. The study aims were (i) to investigate the effects of middle-ear stiffness on the auditory brainstem neural encoding of phase in human adults with normal pure-tone thresholds and (ii) to investigate the relationships between middle-ear stiffness-induced changes in wideband acoustic immittance and neural encoding of phase. The auditory brainstem neural encoding of phase was measured using the auditory steady-state response (ASSR) with and without middle-ear stiffness elicited via contralateral activation of the middle-ear muscle reflex (MEMR). Middle-ear stiffness was quantified using a wideband acoustic immittance assay of acoustic absorbance. Statistical analyses demonstrated decreased ASSR phase lag and decreased acoustic absorbance with contralateral activation of the MEMR, consistent with increased middle-ear stiffness changing the auditory brainstem neural encoding of phase. There were no statistically significant correlations between stiffness-induced changes in wideband acoustic absorbance and ASSR phase. The findings of this study may have important implications for understanding binaural cue sensitivity and horizontal plane sound localization in audiologic and otologic clinical populations that demonstrate changes in middle-ear stiffness, including cochlear implant recipients who use combined electric and binaural acoustic hearing and otosclerosis patients.
Similar content being viewed by others
Introduction
This study investigated the effects of increased middle-ear stiffness on the auditory brainstem neural encoding of phase using clinically translatable, non-invasive assays in human adults with normal pure-tone threshold sensitivity. The middle ear is responsible for transmitting mechanical sound waves from the low-impedance air-filled space of the outer and middle ear to the high-impedance fluid-filled cochlea [1]. Changes in middle-ear forward transmission, such as increased stiffness and mass, can alter the acoustic properties of sound arriving at the cochlea. Using a three-dimensional finite element model, O’Connor et al. [2] showed that increased tympanic membrane (TM) stiffness attenuated low-frequency acoustic transmission and contributed to decreased middle-ear transmission group delay, while increasing the mass of the TM resulted in increased group delay.
The transmission of phase information is one aspect of sound that can be altered by increased middle-ear stiffness [3,4,5]. Phase is defined as a parameter of the acoustic sine wave that provides information related to the proportion of the periodic waveform, or cycle, that has been completed. The phase of an acoustic stimulus is encoded by each ear and contributes to monaural pitch (e.g., [6, 7]) and signal in noise percepts (e.g., [8]). The difference in the phase of the sound arriving at the two ears is the interaural phase difference (IPD) and is thought to contribute to sound localization in azimuth (e.g., [9]). The auditory system is sensitive to interaural phase conveyed by the temporal fine structure and by the temporal envelope of an acoustic stimulus (e.g., [10, 11]). In normal-hearing listeners, such cochlear encoding of fine-structure phase at the group level is altered by an average of 30° when middle-ear stiffness increases due to activation of the middle-ear muscle reflex (MEMR, [3,4,5]).
The auditory steady-state response (ASSR), an auditory evoked potential that represents phase-locked synchronous neural firing to the envelope of an acoustic stimulus, can be used to assess middle-ear stiffness effects on the auditory neural encoding of phase. While the temporal fine structure conveys interaural information primarily for lower frequency acoustic stimuli, interaural differences in the stimulus envelope contribute to horizontal plane sound localization for higher frequency acoustic stimuli [10, 11]. Many cochlear implant (CI) recipients only have access to interaural cues conveyed by the stimulus envelope (e.g., [10, 12]), further highlighting the importance of understanding middle-ear stiffness effects on the neural encoding of stimulus envelope phase.
Two patient populations that can demonstrate changes in middle-ear stiffness are CI recipients and otosclerosis patients. Recent work showed decreased absorbance of low-frequency acoustic power into the middle-ear space, consistent with increased middle-ear stiffness, following CI surgery [13,14,15] that persists through at least 6 months post-activation of the implant [16]. Increased middle-ear stiffness after placement of a cochlear implant may alter the transmission and encoding of acoustic information by an implanted ear with preserved acoustic hearing, leading to potential differences in the encoding of low-frequency acoustic phase information between ears in the sub-population of CI recipients who use combined electric and binaural acoustic (EAS) listening. Middle-ear stiffness-induced differences in the encoding of phase between ears may contribute to the poor horizontal plane sound localization abilities observed in some CI EAS listeners [12, 17,18,19,20].
Otosclerosis is an audiologic disorder characterized by increased stiffness of the ossicular chain in the middle-ear cavity due to sclerotic bone growth and fixation of the stapedial footplate about its anterior annulus [21]. Otosclerosis can result in differing degrees of middle-ear stiffness between left and right ears, potentially leading to differences in phase encoding between ears that may impact functional spatial hearing. In fact, Redfors et al. [22] found that patients with otosclerosis self-reported difficulties with spatial hearing and sound localization that persisted even in those treated with hearing aids.
Understanding the effects of increased middle-ear stiffness on the neural encoding of phase in a normal auditory system will form a basis for future work to understand the effects of stiffness-induced changes in clinical populations. The aims of this study were twofold: (i) to investigate the effects of increased middle-ear stiffness on ASSR phase elicited by tonebursts presented separately at 3 stimulus levels in adults aged 18 to 50 years with normal pure-tone thresholds and (ii) to investigate correlations between middle-ear stiffness-induced changes in wideband acoustic immittance (WAI) measures (i.e., acoustic absorbance and power transmittance) and ASSR phase. We hypothesized that increased middle-ear stiffness would decrease ASSR phase with larger effects observed for higher ASSR stimulus levels and that positive correlations would be observed between middle-ear stiffness-induced changes in WAI measures and stiffness-induced changes in ASSR phase.
Methods and Materials
Participant Demographics and Study Inclusion Criteria
Forty-three adults (32 females and 11 males; 38 White, 3 Asian, 2 Hispanic/Latinx) between 18 and 50 years of age participated in this study. Seven participants were excluded after completing baseline audiometric testing because they did not meet the criteria for normal pure-tone thresholds (defined below). Four additional participants were excluded because their ASSR data were acquired using a shorter duration toneburst stimulus. Data from 1 final participant were excluded because they decided not to return for ASSR testing after completing baseline audiometric testing due to concerns related to the COVID-19 pandemic. Thus, the final participant cohort included data from 31 participants (24 females and 7 males; 27 White, 3 Asian, 1 Hispanic/Latinx).
The 50-year age limit employed in this study was selected in order to focus on young and middle-aged adults with normal hearing. Studies of age-related hearing loss have used the 50- to 55-year age limit as the delineation between younger and older adulthood (e.g., Blue Mountains Hearing Study, [23], Baltimore Longitudinal Study of Aging, [24]). Participants were recruited from the Vanderbilt University and Vanderbilt University Medical Center (VUMC) communities and from the greater Nashville, Tennessee area. All study procedures were approved by the VUMC IRB, and participants completed a VUMC IRB-approved consent process (IRB#210138) and were compensated for their time consistent with IRB guidelines. Inclusion criteria for this study included normal pure-tone threshold sensitivity from 0.25 to 8 kHz including 3 and 6 kHz and normal middle-ear function as defined below. Exclusion criteria included any type or degree of hearing loss, a history of traumatic brain injury, or a history of a neurological disorder.
Baseline Testing
All participants completed baseline testing that consisted of (i) pure-tone audiometric threshold testing in standard and extended high-frequency ranges; (ii) tympanometry and ipsilateral and contralateral MEMR testing; (iii) distortion product otoacoustic emission (DPOAE) testing; and (iv) medial olivocochlear reflex (MOCR) testing using transient-evoked (TE) OAEs and contralaterally presented broadband noise (BBN) and click elicitors.
Normal hearing was defined as behavioral pure-tone thresholds ≤ 20 dB hearing level (HL) at octave frequencies from 0.25 to 8 kHz and inter-octave frequencies of 3 and 6 kHz, in each ear. Extended high-frequency thresholds were measured at 9, 10, 11, 12, 14, and 16 kHz, but participants were not excluded based on having elevated extended high-frequency pure-tone thresholds. All behavioral audiometric testing was completed using a calibrated Grason-Stadler Instruments (GSI) 61 audiometer with ER-3A insert earphones (0.25 to 8 kHz) and Sennheiser supra-aural headphones (9 to 16 kHz).
Participants were required to have evidence of a mobile tympanic membrane as evidenced by middle-ear compliance ≥ 0.2 mmho and normal tympanometric peak pressure between − 100 and 100 decapascals for both ears to be included in this study. Participants’ ipsilateral and contralateral MEMR thresholds were measured for both ears using a 226-Hz probe tone and pure-tone elicitor stimuli from 0.5 to 4 kHz and BBN. MEMR threshold was defined as the level at which a change in middle-ear compliance of ≥ 0.02 mmho occurred. This criterion represents the standard clinical protocol for MEMR threshold estimation; however, it may overestimate MEMR threshold (e.g., [5]). MEMR elicitors were presented 5 dB below and 5 dB above MEMR threshold to confirm growth of the MEMR with increased elicitor level. All tympanometry and MEMR testing was completed using the GSI Tympstar middle-ear test system.
DPOAEs were measured at the cubic distortion frequency of 2f1-f2 with f2 primary tones from 0.5 to 8 kHz tested at 2 points per octave. Primary tone levels of L1 = 65 dB SPL and L2 = 55 dB SPL and a frequency ratio of f2/f1 = 1.22 were used. All DPOAE testing was completed using the Mimosa Acoustics HearID hardware and software platforms coupled to an ER-10C probe with a foam ear tip. All participants were required to have present DPOAEs defined as a signal-to-noise ratio (SNR; distortion product – noise floor) ≥ 6 dB for at least 6 out of the 9 frequencies tested for each ear.
MOCR strength was assessed using a TEOAE and contralateral MOCR activator paradigm. TEOAEs were elicited by 100-μs click stimuli presented at 65 dB peak SPL (pSPL). All click stimuli were presented at the same level and the same polarity (sometimes referred to as a “linear” click paradigm). The MOCR was activated by 2 different elicitors, continuous BBN and clicks at a rate of 20/s, presented contralaterally at 60 and 65 dB SPL (BBN) and 60 and 65 dB pSPL (clicks). All MOCR data were acquired using the Intelligent Hearing Systems USB hardware and SmartTrOAE software coupled to an ER-10D OAE probe with a foam ear tip. The order of MOCR conditions for click and BBN elicitors was (i) without contralateral elicitor (test), (ii) with contralateral elicitor (test), (iii) without contralateral elicitor (retest), and (iv) with contralateral elicitor (retest). Whether participants received the click or BBN elicitor first was counterbalanced among participants.
The Suppression Analysis Module in the SmartTrOAE software was used to analyze the MOCR data. MOCR strength was quantified for test and retest conditions as the difference in TEOAE amplitude in the 8- to 18-ms post-stimulus time window for responses with versus without the contralateral elicitor. MOCR strength for test and retest conditions were averaged together for all participants and used as the dependent variable for MOCR analyses. Quantifying TEOAE amplitude in the 8- to 18-ms range served 2 purposes: (i) previous studies showed that MOCR strength is maximal in this temporal region [25, 26] and (ii) quantifying TEOAE amplitude between 8 and 18 ms minimized the potential confound of stimulus artifact.
Obtaining measures of MOCR strength with a contralateral BBN elicitor allowed for comparison of the study sample to established metrics of MOCR strength. MOCR testing using contralateral click stimuli as the reflex activator provided data about whether the contralateral stimuli used in the ASSR portion of this study elicited the MOCR. This aided in the interpretation of the findings of this study by allowing for differentiation between MOCR and MEMR effects on the auditory brainstem neural encoding of phase.
ASSR Recording Parameters
All ASSR data were acquired using the Intelligent Hearing Systems USB hardware and Advanced Auditory Research Module (AARM) software coupled to an ER-3A insert earphone with a foam ear tip. Data were analyzed using the “Spectral Analysis” feature in the AARM software which provided polar and spectral plots of the data as well as the phase and amplitude of the physiologic response for spectral bins from 0 to 19.995 kHz with a spectral resolution of 4.89 Hz (n = 4096 total spectral bins).
ASSRs were recorded using silver/silver-chloride or gold surface disc electrodes and a two-channel electrode montage (ipsilateral channel: Cz to Aipsi; contralateral channel: Cz to Acontra; ground: Fpz, using international 10–20 system electrode position references). All electrode impedances were maintained below 5 kOhms throughout the duration of testing. Electroencephalographic (EEG) activity was amplified 100,000 times, high-pass filtered at 30 Hz, and low-pass filtered at 300 Hz. Each ASSR sweep consisted of a sequence of 15 0.5-kHz Blackman-windowed toneburst presentations. Each individual 0.5-kHz toneburst in the sequence was 8 ms in duration, and tonebursts were presented at a rate of 78.125 Hz with a sampling rate of 20 kHz. ASSR sweeps were averaged together for a total of at least 4 min of collection time that resulted in at least 1160 sweeps per recording. ASSR phase and amplitude measures were derived based on the average of at least 1160 sweeps (i.e., toneburst sequences) as opposed to being based on the number of individual toneburst presentations. The decision to utilize a toneburst presentation rate within the context of toneburst sequences rather than using amplitude modulated tones to elicit the ASSR is supported by findings from Dobie and Wilson [27] who demonstrated no difference in ASSRs generated by toneburst presentation rates and amplitude-modulated tones in adults with normal hearing. See Fig. 1a, b for a comparison of the toneburst sequence paradigm used in this study to a standard toneburst repetition rate paradigm.
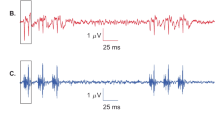
Comparison of the toneburst repetition rate ASSR acquisition paradigm (a) to the toneburst sequence ASSR acquisition paradigm (b). The toneburst repetition rate illustration represents 60 tonebursts presented at a constant rate of 78.125 Hz. ASSR phase and amplitude measures are acquired at the repetition rate of all 60 tonebursts. The toneburst sequence acquisition illustration represents 4 toneburst sequences containing 15 tonebursts in each sequence. The tonebursts in each sequence are presented at a rate of 78.125 Hz with an inter-stimulus interval (ISI; 12.8 ms) between each sequence. ASSR phase and amplitude are measured at the 78.125-Hz toneburst repetition rate for each toneburst sequence, and the responses from each sequence are averaged together. Representation of the ASSR elicited by the toneburst sequence paradigm analyzed in the temporal domain (c) and spectral domain (d) is shown for 1 representative participant (ME_Phase x0HOaA). Blue arrow heads in c highlight the 15 distinct peaks in the temporal waveform elicited by the 15 tonebursts in the toneburst sequence
Figure 1c, d shows examples of ASSR analyses employed in this study for 1 representative participant (ME_Phase x0HOaA). ASSR data are represented in the temporal domain (Fig. 1c) and the spectral domain (Fig. 1d). Note the 15 distinct peaks representing neural responses generated by the 15 tonebursts included in the toneburst sequence when the ASSR is analyzed in the temporal domain (Fig. 1c). Additionally, a robust spectral peak was observed at the 78.125-Hz fundamental frequency, and a secondary spectral peak was observed at the 156.25-Hz harmonic frequency demonstrating successful elicitation of the ASSR by the toneburst sequence paradigm (Fig. 1d). The phase and amplitude of the ASSR was obtained for the 78.125-Hz spectral region. These phase and amplitude measures were used to calculate the slope and shift values as described below which served as the dependent variables for statistical analyses.
The 78.125-Hz toneburst presentation rate was selected to maximize contributions from rostral auditory brainstem structures in the generation of the physiologic response (e.g., Kuwada et al. [28], but see [29]. This study employed a 0.5-kHz toneburst frequency because increased middle-ear stiffness primarily affects lower frequencies. Additionally, unpublished data from the Auditory Physiology Laboratory (PI: Linda J. Hood, PhD) at VUMC showed that WAI-MEMR magnitude is largest for 0.5- and 0.7-kHz half-octave bands. The decision to use a 0.5-kHz ASSR stimulus rather than 0.7 kHz was based on increasing the clinical application of the findings of this study. Specifically, CI EAS patients are more likely to have acoustic hearing preservation in the lower audiometric frequencies (i.e., 0.5 kHz and below). Thus, the methodology developed by this study can be applied by future studies including CI EAS patients.
Noise in the physiologic recordings was estimated using the average amplitude of electrical activity for the 6 spectral bins immediately below and above the target spectral bin (78.125 Hz). Thus, noise was estimated based on the 12 spectral bins from 48.83 to 107.42 Hz, excluding the 78.125-Hz spectral bin. The SNR of each ASSR recording was calculated by comparing the response amplitude at the target frequency (78.125 Hz) to the amplitude of the averaged noise estimate (20 × log[Amptarget / Ampnoise]). ASSR recordings were required to have a dB SNR value ≥ 6 dB in order to be included in further analyses.
Aim I: Middle-ear Stiffness and ASSR Recordings
In this study, contralaterally elicited MEMR was used to increase middle-ear stiffness in the test ear. Changes in acoustic absorbance and power transmittance were measured using WAI to quantify the effects of middle-ear stiffness. Fast-rate ASSRs quantified the neural encoding of phase primarily through the level of the auditory brainstem (Kuwada et al. [28]), though higher-order cortical structures may also have contributed to the ASSR (e.g., [29]). ASSRs were compared with and without contralateral elicitation of the MEMR in order to quantify the effects of increased middle-ear stiffness on the auditory brainstem neural encoding of phase. ASSRs were elicited using a toneburst sequence paradigm (Fig. 1b) at stimulus levels of 75, 80, and 85-dB pSPL in the right ear of all participants. Increased middle-ear stiffness in the ASSR test ear (right) was elicited by presenting an MEMR elicitor to the contralateral ear (left) for every participant. To elicit the MEMR, 100-μs click stimuli with a presentation rate of 20/s were presented to the left ear at levels from 60 to 90-dB pSPL in 5-db steps [30]. ASSRs were acquired with and without the MEMR elicitor at each level for a total of 8 ASSR recordings for each of the 3 ASSR stimulus levels. The order in which ASSR stimulus levels were acquired and MEMR elicitor levels were presented was randomized among participants.
The click MEMR elicitor used in this study was selected in order to minimize elicitation of the MOCR as much as possible. Johnsen and Terkildsen [30] showed that click stimuli presented contralaterally at rates from 8 to 128/s elicited the MEMR in human adults with normal hearing. Additionally, contralateral click rates ≤ 20/s did not elicit a measurable MOCR [31, 32]. Thus, the findings of Johnsen and Terkildsen [30], Veuillet et al. [32], and Boothalingam and Purcell [31] support the use of contralateral click stimuli at 20/s to elicit the MEMR [30] while minimizing elicitation of the MOCR [31, 32] in adults with normal hearing.
Aim II: Correlations Between WAI Measures and ASSR Phase
All WAI data acquisition was completed using the Mimosa Acoustics HearID hardware and software platforms coupled to an ER-10C probe with a foam ear tip. WAI recordings were acquired in the right ear using 70-dB SPL chirp stimuli with and without 100-μs click stimuli presented at a rate of 20/s in the left ear from 60 to 90-dB pSPL in 5-dB steps. Acoustic absorbance and power transmittance measures were acquired from 0.21 to 6 kHz. Stiffness-induced changes in acoustic absorbance and power transmittance were calculated for a half-octave band with a 0.5-kHz center frequency (range of 421.88 to 585.94 Hz). Furthermore, a 0.5-kHz half-octave band was selected for WAI analyses in order to provide overlap with the spectral composition of the 0.5-kHz ASSR toneburst used in Aim I.
All stimuli used for testing in this study were calibrated using a Bruel & Kjaer Pulse calibration system Type 3560C with Type 4157 coupler to increase study accuracy and reproducibility. Biologic listening checks and calibrations of all equipment were completed before each test session. All physiologic data were analyzed and reviewed by 2 individuals experienced in the analysis of auditory physiologic responses to increase the validity of acquired data. Based on the objective nature of ASSRs, the expert review focused on the quality of the ASSR data rather than on determining the presence of the ASSR.
Statistical Analyses
For Aim I analyses, ASSR phase and amplitude were quantified for each recording and were plotted as a function of MEMR elicitor level separately for each of the 3 ASSR stimulus levels. A linear regression model was generated for each condition for each participant using the “lm” function from the R base package (version 3.6.0). For each ASSR stimulus level, participants were required to have ASSR data with an amplitude of ≥ 0.4 μV and an SNR of ≥ 6 dB for at least 3 out of the 7 conditions with presentation of the MEMR elicitor in order to be included in analyses.
Potential outlier data points in the linear regression models for each participant were evaluated by plotting (i) residual vs. fitted plots, (ii) normal Q-Q plots, (iii) scale location plots, and (iv) residual vs. leverage plots for each condition. There are many methods for determining statistical outliers in a dataset. Using 4 different outlier analyses provided a more thorough identification of potential outliers.
All potential outlier data points were reviewed to ensure accurate data input and to ensure that they met the ASSR amplitude and SNR criteria. There was no justification for removing potential outlier data points. Thus, a robust linear regression model was generated for each participant using the “rlm” function from the “MASS” package in R. Robust linear regression adjusts the fit of the linear regression line for potential outliers by weighting the residuals. Using robust linear regression, data points with a larger residual are assigned a lower weight in the calculation of the linear regression line. The slopes of the robust linear regression lines for each participant were obtained from the models and used as the dependent variable for statistical analyses. A negative phase slope value indicated decreased ASSR phase with increased MEMR elicitor level (consistent with increased middle-ear stiffness).
We observed a high degree of between-participant variability in baseline ASSR phase values recorded without presentation of the MEMR elicitor. To be able to reference differences among test conditions to individual participants, ASSR phase shifts and amplitude shifts were calculated by subtracting ASSR phase or amplitude with contralateral presentation of the MEMR elicitor from ASSR phase or amplitude without contralateral presentation of the MEMR elicitor in the same subject. The ASSR phase shift measures quantified middle-ear stiffness-induced changes in ASSR phase normalized for each individual participant to the condition without the MEMR elicitor. To aid in the interpretation of findings, we also calculated the corresponding stiffness-induced temporal delays based on the measured ASSR phase shifts using the following formula:
where Td indicates the temporal delay, ps indicates the ASSR phase shift in degrees, and p indicates the period of the 0.5-kHz ASSR stimulus frequency in ms.
The primary goal of the first aim was to determine whether middle-ear stiffness affected the auditory brainstem neural encoding of phase. Four dependent variables were available for statistical analyses: (i) ASSR phase slope, (ii) ASSR amplitude slope, (iii) ASSR phase shift, and (iv) ASSR amplitude shift. One-sample t tests were calculated using the “t.test” function from the base R package to determine whether the group mean of each dependent variable differed significantly from zero. A slope or shift value that differed significantly from zero indicated statistically significant effects of middle-ear stiffness on the auditory brainstem neural encoding of phase. p values were corrected for multiple comparisons using the false discovery rate when multiple comparisons were employed.
Additionally, Pearson correlation coefficients were calculated to examine the effects of participant age on stiffness-induced changes on ASSR phase slope for each ASSR stimulus level using the “cor.test” function in R. The effects of ASSR stimulus level on stiffness-induced changes in ASSR phase slope also were investigated for the cohort of participants with ASSR data for all 3 stimulus levels (n = 19). A one-way repeated measures ANOVA with post hoc pairwise t tests with pooled standard deviation corrected for multiple comparisons using the Bonferroni correction was calculated using the “aov” and “pairwise.t.test” function in R to assess for differences in ASSR phase slopes among stimulus levels.
For Aim II, acoustic absorbance and power transmittance were plotted as a function of MEMR elicitor level and a linear regression model was generated using the “lm” function from the base R package. Potential outliers were identified and reviewed using the same procedures described for Aim I. A robust linear regression model was generated using the “rlm” function from the “MASS” R package in order to adjust the fit of the linear regression line for potential outlier data points. The slopes of the robust linear regression lines were obtained for each participant and used as the dependent variable for correlational analyses described below. A negative acoustic absorbance and power transmittance slope indicated decreased absorbance and transmittance with increased MEMR elicitor level consistent with contralateral activation of the MEMR increasing middle-ear stiffness.
The primary goal of the second aim was to investigate correlations between middle-ear stiffness-induced changes in WAI measures and ASSR phase. Correlations between the following variable pairs were investigated: (i) ASSR phase slope and acoustic absorbance slope and (ii) ASSR phase slope and power transmittance slope. Pearson correlation coefficients for these variable pairs were calculated using the “cor.test” function from the base R package.
Participant Cohorts and Power Analyses
Of the 31 participants meeting the overall inclusion criteria for this study, 3 cohorts were derived based on ASSR data meeting amplitude and SNR criteria. For each of the 3 ASSR stimulus levels (75-, 80-, and 85-dB pSPL), each participant completed 7 ASSR recordings with contralateral presentation of the MEMR elicitor at levels ranging from 60 to 90-dB pSPL in 5-dB steps. Participants were required to have ASSR data meeting amplitude and SNR criteria described above for ≥ 3 ASSR recordings with presentation of the MEMR elicitor. These requirements were implemented in order for a linear regression line to be fit based on at least 3 ASSR data points. The composition of each cohort included 28 participants meeting inclusion criteria for the 85-dB ASSR stimulus level, 24 participants meeting inclusion criteria for the 80-dB ASSR stimulus level, and 20 participants meeting inclusion criteria for the 75-dB ASSR stimulus level.
Post hoc power analyses were calculated for the Aim I ASSR phase slope analyses outlined above related to the investigation of middle-ear stiffness effects on the auditory brainstem neural encoding of phase. Power analyses were calculated for one-sample t tests using the “pwr.t.test” function from the “pwr” package in R. Power (95 %) was achieved for the 75-dB ASSR stimulus level cohort; 54 % power was achieved for the 80-dB ASSR stimulus level cohort, and 64 % power was achieved for the 85-dB stimulus level cohort. Based on the magnitude of the effect size and between-participant variability observed in this study, future sample sizes of 41 and 43 participants would be required for the 85-dB and 80-dB ASSR stimulus level cohorts, respectively, in order to achieve 80 % statistical power. Recruiting additional participants to increase statistical power for the 80- and 85-dB ASSR stimulus level cohorts was not feasible given the limitations associated with the COVID-19 global pandemic during data collection for this study.
Results
Baseline Auditory Function
Data are presented for each ASSR stimulus level cohort (75, 80, and 85 dB) individually in this section and the sections to follow. Group mean pure-tone thresholds were within the normal range (≤ 20-dB HL) from 0.25 to 8 kHz and were within the near-normal range (≤ 25-dB HL) from 9 to 16 kHz for both ears of all ASSR stimulus level cohorts. Differences in group mean pure-tone thresholds between ears and among cohorts did not exceed 5 dB, which is widely accepted as within test–retest repeatability. These behavioral pure-tone thresholds indicate normal pure-tone threshold sensitivity for both ears among all participants for the investigation of middle-ear stiffness and the auditory brainstem neural encoding of phase.
Ipsilateral and contralateral MEMR thresholds were elicited by 0.5, 1, 2, and 4-kHz pure tones and BBN in order to assess baseline MEMR function. Group mean ipsilateral MEMR thresholds were within the expected normal range (≤ 95-dB HL; e.g., [33]) across all elicitor conditions for both ears. Contralateral MEMR thresholds were elevated (i.e., poorer) compared to ipsilateral thresholds for all conditions. Compared to pure-tone elicitors, BBN elicited lower (i.e., better) ipsilateral and contralateral MEMR thresholds for both ears.
WAI-MEMR: Quantifying Middle-ear Stiffness
Acoustic absorbance and power transmittance were measured in the right ear of all participants with and without an MEMR elicitor (20/s click stimuli) presented contralaterally in the left ear. This was then used as an indication that the contralateral stimulus used during ASSR testing increased middle-ear stiffness in the test ear. A decrease in low-frequency acoustic absorbance and power transmittance with presentation of the MEMR elicitor indicated increased middle-ear stiffness. Figure 2 shows acoustic absorbance for a half-octave band centered at 0.5 kHz as a function of MEMR elicitor level for the 3 ASSR stimulus level cohorts. Note that the number of participants included for each ASSR stimulus level differs (see “Methods and Materials” section and Fig. 2 legend for details). Data for power transmittance are not shown in the figure as the results were similar to acoustic absorbance.
Line plots with linear regression lines showing group mean (± 1 standard error, SE) acoustic absorbance as a function of MEMR elicitor levels. The slope of the robust linear regression line for each individual participant was the dependent variable used for one-sample t test statistical analyses. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (red circles); 24 participants were included in the 80-dB ASSR stimulus level cohort (blue circles); and 20 participants were included in the 75-dB ASSR stimulus level cohort (black circles)
Acoustic absorbance and power transmittance for the half-octave band centered at 0.5 kHz decreased with increasing MEMR elicitor level for all 3 cohorts. The slopes of the robust linear regression lines demonstrated a statistically significant 0.8 % decrease in acoustic absorbance for every 10-dB pSPL increase in MEMR elicitor level for the 85-dB ASSR stimulus level cohort (slope = − 0.08, R2(26) = 0.60, t(27) = − 8.88, P = 5.1 × 10−9) and statistically significant 0.9 % decreases in acoustic absorbance for every 10-dB pSPL increase in MEMR elicitor level for the 80-dB and 75-dB ASSR stimulus level cohorts (80-dB cohort: slope = − 0.09, R2(22) = 0.62, t(23) = − 8.58, P = 2 × 10−8; 75-dB cohort: slope = − 0.09, R2(18) = 0.61, t(19) = − 7.43, P = 4.9 × 10×7). There were no differences in the slopes of the acoustic absorbance functions among the 3 ASSR stimulus level cohorts (one-factor ANOVA: F(2) = 0.017, P = 0.983). Additionally, the slope analyses showed a statistically significant 0.2-dB decrease in power transmittance for every 10-dB pSPL increase in MEMR elicitor level for all 3 ASSR stimulus level cohorts (85-dB cohort: slope = − 0.02, R2(26) = 0.60, t(27) = − 6.49, P = 1.7 × 10−6; 80-dB cohort: slope = − 0.02, R2(22) = 0.62, t(23) = − 6.08, P = 5.1 × 10−6; 75-dB cohort: slope = − 0.02, R2(18) = 0.61, t(19) = − 5.43, P = 3 × 10−5).
Differentiating MEMR and MOCR Contributions to Middle-ear Stiffness
The contralateral MOCR pathway was activated separately by 2 elicitors, continuous BBN and 20/s click stimuli, presented at 2 levels (60 and 65-dB SPL for BBN and 60 and 65-dB pSPL for clicks) to assess whether the contralateral stimulus used to elicit middle-ear stiffness in this study also elicited the MOCR. MOCR strength across elicitor conditions for the 3 ASSR stimulus level cohorts is shown in Fig. 3a–c.
Bar plots showing group mean (error bars show SE) contralateral MOCR strength elicited by BBN (orange bars) and 20/s click stimuli (blue bars). One-sample t tests were used to determine whether TEOAE amplitude reduction differed from zero indicating an MOCR. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (a), 24 participants were included in the 80-dB ASSR stimulus level cohort (b), and 20 participants were included in the 75-dB ASSR stimulus level cohort (c)
The contralateral presentation of the BBN elicitor at 60 and 65-dB SPL resulted in statistically significant reductions in TEOAE amplitude for all cohorts, consistent with the presence of MOCR activation in these participant samples (orange bars in Fig. 3; 85-dB cohort with 60-dB SPL BBN: t(27) = 4.87, P = 1.3 × 10−4; 80-dB cohort with 60-dB SPL BBN: t(23) = 4.09, P = 4.5 × 10−4; 75-dB cohort with 60-dB SPL BBN: t(19) = 4.69, P = 2.4 × 10−4; 85-dB cohort with 65-dB SPL BBN: t(27) = 7.04, P = 4.2 × 10−7; 80-dB cohort with 65-dB SPL BBN: t(23) = 6.70, P = 1.2 × 10−6; 75-dB cohort with 65-dB SPL BBN: t(19) = 5.78, P = 1.4 × 10−5). In contrast, no significant reductions in TEOAE amplitude were observed with contralateral presentation of the click elicitor for any condition (blue bars in Fig. 3; 85-dB cohort with 60-dB pSPL clicks: t(27) = 0.57, P = 0.57; 80-dB cohort with 60-dB pSPL clicks: t(23) = 0.67, P = 0.51; 75-dB cohort with 60-dB pSPL clicks: t(19) = − 0.12, P = 0.90; 85-dB cohort with 65-dB pSPL clicks: t(27) = 0.60, P = 0.55; 80-dB cohort with 65-dB pSPL clicks: t(23) = − 0.32, P = 0.75; 75-dB cohort with 65-dB pSPL clicks: t(19) = 0.60, P = 0.56). The minimal impact of contralateral click stimuli on TEOAE amplitude is consistent with minimal to no co-activation of the MOCR by the stimulus used to elicit middle-ear stiffness in these participant samples.
Middle-ear Stiffness and ASSR Phase
Group mean ASSR SNRs remained constant across MEMR elicitor levels, and no differences were observed among participant cohorts. Additionally, the group mean SNR values across conditions and participant cohorts were well above the 6-dB SNR inclusion criterion used in this study, providing evidence of robust physiologic responses for the analysis of the effects of middle-ear stiffness on the auditory brainstem neural encoding of phase (additional details are provided in Fig. 4).
Line plots showing group mean (± 1 SE) ASSR SNR across ASSR stimulus levels and MEMR elicitor levels. Data plotted at 55 dB represent the no contralateral MEMR elicitor condition. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (red circles), 24 participants were included in the 80-dB ASSR stimulus level cohort (blue squares), and 20 participants were included in the 75-dB ASSR stimulus level cohort (black diamonds)
ASSR phase was measured at the toneburst presentation rate of 78.125 Hz with and without contralateral presentation of the MEMR elicitor to assess the impact of middle-ear stiffness on the auditory brainstem neural encoding of phase. ASSR phase as a function of MEMR elicitor level is shown in Fig. 5a. Group mean ASSR phase decreased with increasing MEMR elicitor level for all ASSR stimulus level cohorts. Robust linear regression slope analyses demonstrated that for every 10-dB pSPL increase in MEMR elicitor level, ASSR phase decreased by 2.8° for the 85-dB ASSR stimulus level cohort (slope = − 0.28, R2(26) = 0.24, t(27) = − 2.36, P = 0.039), ASSR phase decreased by 3.7° for the 80-dB ASSR stimulus level cohort (slope = − 0.37, R2(22) = 0.34, t(23) = − 2.16, P = 0.04), and ASSR phase decreased by 6.5° for the 75-dB ASSR stimulus level cohort (slope = − 0.65, R2(18) = 0.27, t(19) = − 3.75, P = 0.0042).
(a) Scatter plots with linear regression lines showing group mean (± 1 SE) ASSR phase across MEMR elicitor levels. The slope of the robust linear regression lines for each individual participant was the dependent variable used for one-sample t test statistical analyses. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (red circles); 24 participants were included in the 80-dB ASSR stimulus level cohort (blue circles); and 20 participants were included in the 75-dB ASSR stimulus level cohort (black circles). Group mean data (± 1 SE) for ASSR phase without contralateral activation of the MEMR are shown by the square data points to the left of the plot. (b) Group mean (± 1 SE) ASSR phase shifts across MEMR elicitor levels for the 3 ASSR stimulus level cohorts. One-sample t tests were used to determine whether ASSR phase shifts differed significantly from zero. (c) Corresponding group mean (± 1 SE) temporal delays across MEMR elicitor levels for all cohorts calculated based on ASSR phase shifts
Due to observed between-participant variability in baseline ASSR phase values, phase data were normalized to each participant’s ASSR phase value obtained without the MEMR elicitor. Thus, ASSR phase shifts were calculated by subtracting phase with presentation of the MEMR elicitor from phase without presentation of the MEMR elicitor. Figure 5b shows ASSR phase shift, and Fig. 5c shows the corresponding temporal delay as a function of MEMR elicitor level for the 3 ASSR stimulus level cohorts. Statistically significant ASSR phase shifts and temporal delays occurred at the 2 highest MEMR elicitor levels for the 75-dB ASSR stimulus level cohort (85-dB pSPL MEMR elicitor level: t(16) = 3.52, P = 0.010; 90-dB pSPL MEMR elicitor level: t(13) = 4.08, P = 0.010). The data indicate a maximum 21° ASSR phase shift, consistent with a 0.12-ms temporal delay, for the 75-dB ASSR stimulus level with 85-dB contralateral MEMR elicitor.
Nineteen participants had ASSR data available for all 3 ASSR stimulus levels allowing for within-participant investigation of the effects of ASSR stimulus level on ASSR phase slope. ASSR phase as a function of MEMR elicitor level for the 19 participants is shown in Fig. 6. A one-way repeated measures ANOVA (dependent variable: ASSR phase slope; independent variable: ASSR stimulus level) demonstrated no statistically significant differences among ASSR phase slopes for the 3 ASSR stimulus levels (f(1) = 2.34, P = 0.14). Although no statistically significant differences were noted, lower ASSR stimulus levels tended to have steeper ASSR phase slopes suggestive of a potentially greater effect of middle-ear stiffness on the auditory brainstem neural encoding of phase for lower stimulus levels.
Scatter plots showing within-participant comparison (group mean ± 1 SE) of ASSR phase across MEMR elicitor levels for the group of 19 participants with ASSR phase data at all 3 ASSR stimulus levels (85-dB ASSR stimulus level shown by blue circles, 80-dB ASSR stimulus level shown by orange squares, and 75-dB ASSR stimulus level shown by black diamonds). Linear regression lines are fit to the data for each ASSR stimulus level. A one-way repeated measures ANOVA was used to determine differences in ASSR phase slope among ASSR stimulus levels
Pearson correlation coefficients were calculated to examine the effects of participant age on ASSR phase slope for the 3 ASSR stimulus level cohorts. No statistically significant correlations were observed between participant age and ASSR phase slope (85-dB ASSR stimulus level cohort: r = 0.14, t(26) = 0.73, P = 0.47; 80-dB ASSR stimulus level cohort: r = 0.16, t(22) = 0.75, P = 0.47; 75-dB ASSR stimulus level cohort: r = − 0.02, t(18) = − 0.07, P = 0.94). Figure 7a–c shows scatter plots representing ASSR phase slope as a function of participant age for all cohorts. Note the fewer number of participants in the older age range, especially for the 75-dB ASSR stimulus level cohort. Additionally, between-participant variability in ASSR phase slope occurred across all ages.
Scatter plots with linear regression lines showing the effects of age on ASSR phase slope across ASSR stimulus level cohorts (a–c). Pearson correlation coefficients were used to investigate the linear relationship between variables for each cohort. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (a), 24 participants were included in the 80-dB ASSR stimulus level cohort (b), and 20 participants were included in the 75-dB ASSR stimulus level cohort (c)
Middle-ear Stiffness and ASSR Amplitude
ASSR amplitude as a function of MEMR elicitor level is shown for the 3 ASSR stimulus level cohorts in Fig. 8a. The robust slope analyses confirmed that ASSR amplitude did not change as a function of MEMR elicitor level for any of the 3 ASSR stimulus level cohorts (85-dB ASSR stimulus level cohort: slope = − 0.0001, R2(26) = 0.35, t(27) = − 0.52, P = 0.61; 80-dB ASSR stimulus level cohort: slope = − 0.0001, R2(22) = 0.23, t(23) = − 0.43, P = 0.67; 75-dB ASSR stimulus level cohort: slope = − 0.0001, R2(18) = 0.34, t(19) = − 0.61, P = 0.55).
(a) Scatter plots with linear regression lines showing group mean (± 1 SE) ASSR amplitude as a function of MEMR elicitor levels. The slope of the robust linear regression lines for each individual participant was the dependent variable used for one-sample t-test statistical analyses. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (red circles); 24 participants were included in the 80-dB ASSR stimulus level cohort (blue circles); and 20 participants were included in the 75-dB ASSR stimulus level cohort (black circles). (b) Group mean (± 1 SE) ASSR amplitude shifts vs. MEMR elicitor levels for the 3 ASSR stimulus level cohorts. One-sample t tests were used to determine whether ASSR amplitude shifts differed significantly from zero
ASSR amplitude shifts were calculated for each MEMR elicitor level, normalizing each participant’s ASSR amplitude to their baseline amplitude without the MEMR elicitor. Figure 8b shows ASSR amplitude shift across MEMR elicitor levels for each of the 3 ASSR stimulus level cohorts. Group mean amplitude shifts did not exceed 0.02 μV and remained constant across MEMR elicitor levels. Slightly greater shifts occurred for the higher ASSR stimulus levels. One-sample t tests showed statistically significant amplitude shifts with MEMR elicitor presentation for the 2 highest ASSR stimulus level cohorts only (85-dB ASSR stimulus level with 90-dB pSPL MEMR elicitor: t(24) = − 2.77, P = 0.013; 85-dB ASSR stimulus level with 85-dB pSPL MEMR elicitor: t(22) = − 3.45, P = 0.0068; 85-dB ASSR stimulus level with 75-dB pSPL MEMR elicitor: t(24) = − 2.91, P = 0.013; 85-dB ASSR stimulus level with 70-dB pSPL MEMR elicitor: t(23) = − 3.36, P = 0.0068; 85-dB ASSR stimulus level with 65-dB pSPL MEMR elicitor: t(22) = − 2.48, P = 0.02; 80-dB ASSR stimulus level with 85-dB pSPL MEMR elicitor level: t(21) = − 3.12, P = 0.0052; 80-dB ASSR stimulus level with 70-dB pSPL MEMR elicitor: t(18) = − 3.85, P = 0.0032; 80-dB ASSR stimulus level with 65-dB pSPL MEMR elicitor: t(16) = − 3.66, P = 0.0032). No statistically significant amplitude shifts occurred for the 75-dB ASSR stimulus level cohort.
Correlations Between WAI and ASSR Measures
The relationship between ASSR phase slope and 0.5-kHz-centered acoustic absorbance slope is shown in Fig. 9a–c, and the relationship between ASSR phase slope and 0.5-kHz-centered power transmittance slope is shown in Fig. 10a–c for the 3 ASSR stimulus level cohorts. Pearson correlation coefficients demonstrated no statistically significant correlations between acoustic absorbance slope and ASSR phase slope for any of the ASSR stimulus level cohorts (85-dB ASSR stimulus level cohort: r = 0.33, t(26) = 1.77, P = 0.09; 80-dB ASSR stimulus level cohort: r = − 0.14, t(22) = 0.68, P = 0.51; 75-dB ASSR stimulus level cohort: r = 0.03, t(18) = 0.14, P = 0.89). Note the trend towards a positive correlation between acoustic absorbance slope and ASSR phase slope for the 85-dB ASSR stimulus level cohort. Additionally, no statistically significant correlations were observed between power transmittance slope and ASSR phase slope for any of the 3 ASSR stimulus level cohorts (85-dB ASSR stimulus level cohort: r = 0.15, t(26) = 0.78, P = 0.45; 80-dB ASSR stimulus level cohort: r = − 0.06, t(22) = − 0.29, P = 0.77; 75-dB ASSR stimulus level cohort: r = − 0.15, t(18) = − 0.65, P = 0.53).
Scatter plots with linear regression lines showing the relationship between ASSR phase slope and acoustic absorbance slope for the 3 ASSR stimulus level cohorts (a–c). Pearson correlation coefficients were used to investigate the linear relationship between variables for each cohort. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (a), 24 participants were included in the 80-dB ASSR stimulus level cohort (b), and 20 participants were included in the 75-dB ASSR stimulus level cohort (c)
Scatter plots with linear regression lines showing the relationship between ASSR phase slope and power transmittance slope for the 3 ASSR stimulus level cohorts (a–c). Pearson correlation coefficients were used to investigate the linear relationship between variables for each cohort. Twenty-eight participants were included in the 85-dB ASSR stimulus level cohort (a), 24 participants were included in the 80-dB ASSR stimulus level cohort (b), and 20 participants were included in the 75-dB ASSR stimulus level cohort (c)
Discussion
This study investigated whether middle-ear stiffness elicited via MEMR activation changed the auditory brainstem neural encoding of phase as measured by ASSR phase and amplitude. As MEMR elicitor level increased, low-frequency acoustic absorbance and ASSR phase decreased. The findings of this study are consistent with the elicitation of middle-ear stiffness via activation of the MEMR which changed the encoding of phase at the auditory brainstem up to a maximum 21° ASSR phase shift consistent with a 0.12-ms temporal delay. Brughera et al. [34] showed human listener ITD thresholds for a 0.5-kHz pure tone ranging from 0.02 to 0.05 ms, indicating that the maximum temporal delay observed in the current study is sufficient to impact behavioral performance on a sound localization task. Furthermore, there was very little to no change in ASSR amplitude as a function of MEMR elicitor level, indicating that middle-ear stiffness appears to change the auditory brainstem neural encoding of phase with minimal impact on neural recruitment and synchrony. This study did not measure the test–retest repeatability of ASSR phase without contralateral activation of the MEMR due to time constraints, and findings should be considered in the context of this potential limitation.
The findings of this study are consistent with other studies that investigated the effects of middle-ear stiffness on the cochlear encoding of phase [3,4,5]. Avan et al. [3] identified an ~ 20° stiffness-induced DPOAE phase shift at 0.6 kHz. Similarly, the present study identified a 21° ASSR phase shift using 0.5-kHz ASSR stimuli presented at 75-dB pSPL. These findings suggest shifts in both the cochlear and neural encoding of phase that occur with comparable magnitude for a similar spectral region. Further within-participant investigations are required in order to make valid comparisons between stiffness-induced effects on phase encoding at the cochlear and neural levels. The stiffness-induced ASSR phase shifts and temporal delays observed in the current study are also consistent with the finite element modeling of O’Connor et al. [2] showing decreased middle-ear transmission group delay with increased TM stiffness.
The following hypothesis related to physiologic mechanisms contributing to stiffness-induced phase changes is proposed. A sinusoidal signal (i.e., phase) is transmitted by the middle-ear system via movements of the ossicular chain. Activation of the MEMR results in contraction of the stapedius muscle and a stiffening of the ossicular chain, which impedes its mobility. A stiffer and less mobile ossicular chain is consistent with reduced transmission of the sinusoid and decreased value of the phase lag coded by the measurement of the ASSR as observed in this study.
This study provides several insights related to physiologic mechanisms that may or may not contribute to middle-ear stiffness-induced phase shifts. First, baseline MOCR and WAI-MEMR testing completed during this study confirmed that the MEMR elicitor activated the MEMR pathway with little to no evidence supporting co-activation of the MOCR pathway (Fig. 3). This supports middle-ear stiffness-induced changes in ASSR phase with minimal to no contribution from activation of the MOCR pathway. The use of contralateral acoustic stimulation to activate the MEMR eliminated the confound of stimulus interactions in the ear canal. Contralateral acoustic stimulation also did not affect ASSR amplitude, which suggests little to no acoustic crossover of the contralateral click stimuli to the cochlea of the test ear. Thus, stimulus interactions in the ear canal and acoustic crossover do not appear to have contributed to the observed effects on ASSR phase demonstrated by this study.
One potential limitation of the linear regression analyses is the between-participant variability in ASSR phase for the 80- and 85-dB ASSR stimulus levels that contributed to functions at the group level that may not have been most accurately fit using a linear model. The between-participant variability in ASSR phase observed in this study is consistent with that observed by Herdman and Stapells [35]. Future studies should investigate other modeling techniques, such as sigmoid curve fitting, that may be a more appropriate fit to group level data for the investigation of middle-ear stiffness effects on brainstem neural phase encoding.
No Effect of Age on Middle-ear Stiffness-induced ASSR Phase Lag
This study did not identify any statistically significant effects of participant age on middle-ear stiffness-induced changes in ASSR phase, potentially indicating consistent effects of middle-ear stiffness on auditory brainstem neural phase encoding across the lifespan from 18 to 50 years. This particular finding should be considered with regard for the limited age range investigated in this study spanning only younger and middle adulthood. Additionally, fewer older participants had available ASSR data meeting inclusion criteria, especially for the 75-dB ASSR stimulus level cohort, and between-participant variability in ASSR phase slope occurred across the lifespan. The fewer number of older participants and variability among participants may have prevented the identification of statistically significant age effects for this study sample.
Correlations Between Measures
Correlation analyses did not demonstrate statistically significant relationships between acoustic absorbance slope and ASSR phase slope or between power transmittance slope and ASSR phase slope, though a trend towards a positive correlation between acoustic absorbance and ASSR phase slope was observed for the 85-dB ASSR stimulus level cohort. The lack of significant correlation between these measures may indicate differential effects of middle-ear stiffness on middle-ear versus auditory brainstem structures.
Additionally, acoustic absorbance and power transmittance slopes may indicate the amount of middle-ear stiffness elicited by the MEMR elicitor. For example, a steeper acoustic absorbance and power transmittance slope is consistent with a greater amount of middle-ear stiffness. A positive correlation between WAI slopes and ASSR phase slope would indicate that greater amounts of middle-ear stiffness partially contribute to greater stiffness-induced ASSR phase shifts. Thus, the lack of correlations may suggest that middle-ear stiffness does not result in additional changes to phase encoding once a threshold amount of middle-ear stiffness is reached.
The use of Pearson correlation coefficients is one potential limitation of these analyses. Acoustic absorbance slope, power transmittance slope, and ASSR phase slope are dependent variables. Correlational analyses comparing multiple dependent variables would be more accurately analyzed using multivariate multiple regression modeling the effects of 1 predictor variable on multiple dependent variables. This study did not have a sufficient number of participants to complete multivariate multiple regression analyses. Thus, the correlational analyses should be considered in the context of the limitations associated with the type of correlation employed.
Impact
The findings of this study have meaningful potential impacts on audiologic practice. Yost [9] demonstrated in adults with normal hearing that an IPD of as little as 5° (~ 28 μs) impacted behavioral IPD discrimination (i.e., minimum audible angle) of a 0.5-kHz pure tone. The current study showed middle-ear stiffness-induced changes in the auditory brainstem encoding of phase consistent with up to a 6.5° decrease in ASSR phase for every 10-dB pSPL increase in MEMR elicitor level and a maximum 21° stiffness-induced ASSR phase shift. These findings emphasize that middle-ear stiffness can result in changes in the auditory brainstem encoding of phase at a magnitude that may alter behavioral sound localization performance.
Recent work suggests increased middle-ear stiffness after placement of a cochlear implant that persists through at least 6 months post-activation [13,14,15,16]. Middle-ear stiffness that differs between ears, whether in unilateral or bilateral CI EAS patients, may result in changes in the brainstem neural encoding of phase that may contribute to larger post-operative IPDs. This may contribute understanding to the variability in binaural cue sensitivity in CI EAS patients who utilize low-frequency acoustic hearing in conjunction with electric hearing [12, 17,18,19,20].
Post-operative middle-ear stiffness in CI recipients is likely due to a combination of factors including changes in middle-ear volume via introduction of the facial recess, sealing of the electrode array at the round window, the presence of the electrode array in the cochlea, and surgical packing around the fenestra with fascia or other grafting material. Middle-ear stiffness in the current study was elicited via activation of the MEMR, which is not likely to be present in the majority of CI recipients due to their degree of sensorineural hearing loss. The application of the findings of this study may have limited generalizability to CI EAS listeners due to differences in mechanisms contributing to middle-ear stiffness. However, the identification of middle-ear stiffness-induced changes in brainstem neural phase encoding provides rationale for the investigation of middle-ear stiffness-induced effects on neural phase encoding in CI EAS listeners.
Additionally, otosclerosis is an audiologic disorder that is characterized by a stiffening of the ossicular chain [21] that can result in differences in middle-ear stiffness between ears. Redfors et al. [22] showed that individuals with otosclerosis self-reported difficulties localizing sounds based on the speech, spatial, and qualities of hearing scale (SSQ) that persisted even in individuals treated with hearing aids. Middle-ear stiffness-induced changes in auditory phase encoding may contribute to difficulties in sound localization in otosclerosis patients.
Conclusion
This study identified a change in the auditory brainstem neural encoding of phase secondary to middle-ear stiffness elicited by activation of the MEMR. ASSR phase decreased by up to 6.5°, and acoustic absorbance decreased by approximately 1 % for every 10-dB pSPL increase in MEMR elicitor level with a maximum 21° stiffness-induced ASSR phase shift. This magnitude of phase shift may be sufficient to alter behavioral performance on a sound localization task. The findings of this study may help to understand the poor spatial hearing abilities of CI EAS listeners and patients with otosclerosis.
References
Merchant SN, Rosowski JJ (2003) Auditory physiology. Surg Ear 59–82
O’Connor KN, Cai H, Puria S (2017) The effects of varying tympanic-membrane material properties on human middle-ear sound transmission in a three-dimensional finite-element model. J Acoustical Soc Am 142(5):2836–2853
Avan P, Büki B, Maat B, Dordain M, Wit HP (2000) Middle ear influence on otoacoustic emissions. I: noninvasive investigation of the human transmission apparatus and comparison with model results. Hear Res 140(1–2):189–201
Büki B, Wit HP, Avan P (2000) Olivocochlear efferent vs. middle-ear contributions to the alteration of otoacoustic emissions by contralateral noise. Brain Res 852(1):140–150
Sun XM (2008) Contralateral suppression of distortion product otoacoustic emissions and the middle-ear muscle reflex in human ears. Hear Res 237(1–2):66–75
Moore BC (2008) The role of temporal fine structure processing in pitch perception, masking, and speech perception for normal-hearing and hearing-impaired people. J Assoc Res Otolaryngol 9(4):399–406
Verschooten E, Shamma S, Oxenham AJ, Moore BC, Joris PX, Heinz MG, Plack CJ (2019) The upper frequency limit for the use of phase locking to code temporal fine structure in humans: a compilation of viewpoints. Hear Res 377:109–121
Hopkins K, Moore BC (2009) The contribution of temporal fine structure to the intelligibility of speech in steady and modulated noise. J Acoustical Soc Am 125(1):442–446
Yost WA (1974) Discriminations of interaural phase differences. J Acoustical Soc Am 55(6):1299–1303
Dietz M, Wang L, Greenberg D, McAlpine D (2016) Sensitivity to interaural time differences conveyed in the stimulus envelope: estimating inputs of binaural neurons through the temporal analysis of spike trains. J Assoc Res Otolaryngol 17(4):313–330
Monaghan JJ, Bleeck S, McAlpine D (2015) Sensitivity to envelope interaural time differences at high modulation rates. Trends Hear 19:2331216515619331
Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF (2014) Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear Res 312:28–37
Merchant GR, Schulz KM, Patterson JN, Fitzpatrick D, Janky KL (2020) Effect of cochlear implantation on vestibular evoked myogenic potentials and wideband acoustic immittance. Ear Hear 41(5):1111–1124
Saoji AA, Shapiro SB, Finley CC, Koka K, Cassis AM (2020) Changes in wide-band tympanometry absorbance following cochlear implantation. Otol Neurotol 41(6):e680–e685
Scheperle RA, Hajicek JJ (2020) Wideband acoustic immittance in cochlear implant recipients: reflectance and stapedial reflexes. Ear Hear 41(4):883–895
Racca JM, Jones LL, Dwyer RT, Ferguson M, Sunderhaus L, Hood LJ, Gifford RH (2022) Changes in acoustic absorbance pre- and post-cochlear implantation. Am J Audiol 1–12
Dunn CC, Perreau A, Gantz B, Tyler RS (2010) Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol 21(1):44–51
Grantham DW, Ashmead DH, Haynes DS, Hornsby BW, Labadie RF, Ricketts TA (2012) Horizontal plane localization in single-sided deaf adults fitted with a bone-anchored hearing aid (Baha). Ear Hear 33(5):595–603
Körtje M, Baumann U, Stöver T, Weissgerber T (2020) Sensitivity to interaural time differences and localization accuracy in cochlear implant users with combined electric-acoustic stimulation. PLoS ONE 15(10):e0241015
Plant K, Babic L (2016) Utility of bilateral acoustic hearing in combination with electrical stimulation provided by the cochlear implant. Int J Audiol 55(sup2):S31–S38
Chole RA, McKenna M (2001) Pathophysiology of otosclerosis. Otol Neurotol 22(2):249–257
Redfors YD, Olaison S, Karlsson J, Hellgren J, Möller C (2015) Hearing-related, health-related quality of life in patients who have undergone otosclerosis surgery: a long-term follow-up study. Int J Audiol 54(2):63–69
Gopinath B, Rochtchina E, Wang JJ, Schneider J, Leeder SR, Mitchell P (2009) Prevalence of age-related hearing loss in older adults: Blue Mountains Study. Arch Intern Med 169(4):415–418
Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM (2011) Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 25(6):763
Berlin CI, Hood LJ, Hurley AE, Wen H, Kemp DT (1995) Binaural noise suppresses linear click-evoked otoacoustic emissions more than ipsilateral or contralateral noise. Hear Res 87(1–2):96–103
Berlin CI, Hood LJ, Wen H, Szabo P, Cecola RP, Rigby P, Jackson DF (1993) Contralateral suppression of non-linear click-evoked otoacoustic emissions. Hear Res 71(1–2):1–11
Dobie RA, Wilson MJ (1998) Low-level steady-state auditory evoked potentials: effects of rate and sedation on detectability. J Acoustical Soc Am 104(6):3482–3488
Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’Angelo WR (2002) Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol 13(04):188–204
Coffey EB, Nicol T, White-Schwoch T, Chandrasekaran B, Krizman J, Skoe E, Kraus N (2019) Evolving perspectives on the sources of the frequency-following response. Nat Commun 10(1):1–10
Johnsen NJ, Terkildsen K (1980) The normal middle ear reflex thresholds towards white noise and acoustic clicks in young adults. Scand Audiol 9(3):131–135
Boothalingam S, Purcell DW (2015) Influence of the stimulus presentation rate on medial olivocochlear system assays. J Acoustical Soc Am 137(2):724–732
Veuillet E, Collet L, Duclaux R (1991) Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65(3):724–735
Silman S, Gelfand SA (1981) The relationship between magnitude of hearing loss and acoustic reflex threshold levels. J Speech Hear Disorders 46(3):312–316
Brughera A, Dunai L, Hartmann WM (2013) Human interaural time difference thresholds for sine tones: the high-frequency limit. J Acoustical Soc Am 133(5):2839–2855
Herdman AT, Stapells DR (2001) Thresholds determined using the monotic and dichotic multiple auditory steady-state response technique in normal-hearing subjects. Scand Audiol 30(1):41–49
Funding
This work was supported by a grant from the Vanderbilt Institute of Clinical and Translational Research (VICTR; grant number VR55626; PI: JMR) and research resources from the Hood Laboratory (PI: LJH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
René H. Gifford is a consultant for Advanced Bionics, Cochlear Limited, Akouos, and Frequency Therapeutics. Linda J. Hood is a consultant for Akouos and Pfizer. These consultant roles are not in conflict with the work reported here. Rafael E. Delgado is employed by and a stock holder of Intelligent Hearing Systems. There are no other conflicts of interest, financial or otherwise, to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Racca, J.M., Delgado, R.E., Gifford, R.H. et al. The Effects of Middle-ear Stiffness on the Auditory Brainstem Neural Encoding of Phase. JARO 23, 859–873 (2022). https://doi.org/10.1007/s10162-022-00872-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-022-00872-0