Abstract
The mammalian cochlea achieves its remarkable sensitivity, frequency selectivity, and dynamic range by spatially segregating the different frequency components of sound via nonlinear processes that remain only partially understood. As a consequence of the wave-based nature of cochlear processing, the different frequency components of complex sounds interact spatially and nonlinearly, mutually suppressing one another as they propagate. Because understanding nonlinear wave interactions and their effects on hearing appears to require mathematically complex or computationally intensive models, theories of hearing that do not deal specifically with cochlear mechanics have often neglected the spatial nature of suppression phenomena. Here we describe a simple framework consisting of a nonlinear traveling-wave model whose spatial response properties can be estimated from basilar-membrane (BM) transfer functions. Without invoking jazzy details of organ-of-Corti mechanics, the model accounts well for the peculiar frequency-dependence of suppression found in two-tone suppression experiments. In particular, our analysis shows that near the peak of the traveling wave, the amplitude of the BM response depends primarily on the nonlinear properties of the traveling wave in more basal (high-frequency) regions. The proposed framework provides perhaps the simplest representation of cochlear signal processing that accounts for the spatially distributed effects of nonlinear wave propagation. Shifting the perspective from local filters to non-local, spatially distributed processes not only elucidates the character of cochlear signal processing, but also has important consequences for interpreting psychophysical experiments.





Similar content being viewed by others
References
Abbas PJ, Sachs MB (1976) Two-tone suppression in auditory-nerve fibers: Extension of a stimulus-response relationship. J Acoust Soc Am 59:112–122
Abdala C, Sininger YS, Ekelid M, Zeng FG (1996) Distortion-product otoacoustic emission suppression tuning curves in human adults and neonates. Hear Res 98:38–53
Altoè A, Shera CA (2020) The cochlear ear horn: Geometric origin of tonotopic variations in auditory signal processing. Sci Rep 10:20528
Altoè A, Shera CA (2020) Nonlinear cochlear mechanics without direct vibration-amplification feedback. Phys Rev Res 2:013218
Altoè A, Charaziak KK, Shera CA (2017) Dynamics of cochlear nonlinearity: Automatic gain control or instantaneous damping? J Acoust Soc Am 142:3510–3519
von Békésy G (1960) Experiments in Hearing, McGraw-Hill, New York
de Boer E (1980) A cylindrical cochlea model: The bridge between two and three dimensions. Hear Res 3:109–131
de Boer E (1981) Short waves in three-dimensional cochlea models: Solution for a block model. Hear Res 4:53–77
de Boer E (1997) Connecting frequency selectivity and nonlinearity for models of the cochlea. Aud Neurosci 3:377–388
de Boer E, Nuttall AL (1999) The inverse problem solved for a three-dimensional model of the cochlea. III. Brushing-up the solution method. J Acoust Soc Am 105:3410–3420
Borges JL (1998) On exactitude in science. In: Collected Fictions, translated by A. Hurley, Viking Penguin, New York, p 325
Brownell WE, Bader CR, Bertrand D, De Ribaupierre Y (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227:194–196
Burda H, Ballast L, Bruns V (1988) Cochlea in old world mice and rats (Muridae). J Morphol 198:269–285
Camalet S, Duke T, Jülicher F, Prost J (2000) Auditory sensitivity provided by self-tuned critical oscillations of hair cells. Proc Natl Acad Sci USA 97:3183–3188
Charaziak KK, Souza P, Siegel JH (2013) Stimulus-frequency otoacoustic emission suppression tuning in humans: comparison to behavioral tuning. J Assoc Res Otolaryngol 14:843–862
Charaziak KK, Dong W, Altoè A, Shera CA (2020) Asymmetry and microstructure of temporal-suppression patterns in basilar-membrane responses to clicks: Relation to tonal suppression and traveling-wave dispersion. J Assoc Res Otolaryngol 21:151–170
Cody A (1992) Acoustic lesions in the mammalian cochlea: Implications for the spatial distribution of the active process. Hear Res 62:166–172
Cooper NP (1996) Two-tone suppression in cochlear mechanics. J Acoust Soc Am 99:3087–3098
Dau T, Püschel D, Kohlrausch A (1996) A quantitative model of the effective signal processing in the auditory system. I. Model structure. J Acoust Soc Am 99:3615–3622
Delgutte B (1990) Physiological mechanisms of psychophysical masking: Observations from auditory-nerve fibers. J Acoust Soc Am 87:791–809
Dewey JB, Applegate BE, Oghalai JS (2019) Amplification and suppression of traveling waves along the mouse organ of Corti: Evidence for spatial variation in the longitudinal coupling of outer hair cell-generated forces. J Neurosci 39:1805–1816
Dong W, Olson ES (2013) Detection of cochlear amplification and its activation. Biophys J 105:1067–1078
Dong W, Olson ES (2016) Two-tone suppression of simultaneous electrical and mechanical responses in the cochlea. Biophys J 111(8):1805–1815
Duifhuis H (1988) Cochlear macromechanics. In: Edelman GM, Gall WE, Cowan WM (eds) Auditory Function: Neurological Bases for Hearing. Wiley, New York, pp 189–211
Fallah E, Strimbu CE, Olson ES (2019) Nonlinearity and amplification in cochlear responses to single and multi-tone stimuli. Hear Res 377:271–281
Fisher JA, Nin F, Reichenbach T, Uthaiah RC, Hudspeth AJ (2012) The spatial pattern of cochlear amplification. Neuron 76:989–997
Fletcher H (1940) Auditory patterns. Rev Mod Phys 12:47
Frank G, Hemmert W, Gummer AW (1999) Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci USA 96:4420–4425
Geisler CD, Yates GK, Patuzzi RB, Johnstone BM (1990) Saturation of outer hair cell receptor currents causes two-tone suppression. Hear Res 44:241–256
Glasberg BR, Moore BCJ (1990) Derivation of auditory filter shapes from notched-noise data. Hear Res 47:103–138
Goblick TJ, Pfeiffer RR (1969) Time-domain measurements of cochlear nonlinearities using combination click stimuli. J Acoust Soc Am 46:924–938
Gorga MP, Neely ST, Kopun J, Tan H (2011) Distortion-product otoacoustic emission suppression tuning curves in humans. J Acoust Soc Am 129:817–827
Green DM (1960) Auditory detection of a noise signal. J Acoust Soc Am 32:121–131
van der Heijden M (2014) Frequency selectivity without resonance in a fluid waveguide. Proc Natl Acad Sci USA 111:14548–14552
Heinz MG, Colburn HS, Carney LH (2002) Quantifying the implications of nonlinear cochlear tuning for auditory-filter estimates. J Acoust Soc Am 111:996–1011
Hudspeth AJ, Jülicher F, Martin P (2010) A critique of the critical cochlea: Hopf—a bifurcation—is better than none. J Neurophysiol 104:1219–1229
Joris PX, Bergevin C, Kalluri R, Mc Laughlin M, Michelet P, van der Heijden M, Shera CA (2011) Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in humans. Proc Natl Acad Sci USA 108:17516–17520
Kalluri R, Shera CA (2001) Distortion-product source unmixing: A test of the two-mechanism model for DPOAE generation. J Acoust Soc Am 109:622–637
Kanis LJ, de Boer E (1993) Self-suppression in a locally active nonlinear model of the cochlea: A quasilinear approach. J Acoust Soc Am 94:3199–3206
Kanis LJ, de Boer E (1994) Two-tone suppression in a locally active nonlinear model of the cochlea. J Acoust Soc Am 96:2156–2165
Kemp DT (1978) Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am 64:1386–1391
Kemp DT, Chum RA (1980) Observations on the generator mechanism of stimulus frequency acoustic emissions two tone suppression. In: van den Brink G, Bilsen FA (eds) Psychophysical Physiological and Behavioural Studies in Hearing. Delft University Press, Delft, pp 39–41
Kemp DT, Chum RA (1980) Properties of the generator of stimulated acoustic emissions. Hear Res 2:213–232
Kuhn TS (1957) The Copernican Revolution: Planetary Astronomy in the Development of Western Thought. Harvard University Press, Cambridge
Kummer P, Janssen T, Arnold W (1995) Suppression tuning characteristics of the 2f1 – f2 distortion-product otoacoustic emission in humans. J Acoust Soc Am 98:197–210
Lighthill J (1981) Energy flow in the cochlea. J Fluid Mech 106:149–213
Lineton B, Wildgoose CMB (2009) Comparing two proposed measures of cochlear mechanical filter bandwidth based on stimulus frequency otoacoustic emissions. J Acoust Soc Am 125:1558–1566
Liu YW, Liu TC (2016) Quasilinear reflection as a possible mechanism for suppressor-induced otoacoustic emission. J Acoust Soc Am 140:4193–4203
Lopez-Poveda EA (2005) Spectral processing by the peripheral auditory system: facts and models. Int Rev Neurobio 70:7–48
Lopez-Poveda EA, Eustaquio-Martin A (2013) On the controversy about the sharpness of human cochlear tuning. J Assoc Res Otolaryngol 14:673–686
Manley GA, van Dijk P (2016) Frequency selectivity of the human cochlea: Suppression tuning of spontaneous otoacoustic emissions. Hear Res 336:53–62
Maxwell BN, Richards VM, Carney LH (2020) Neural fluctuation cues for simultaneous notched-noise masking and profile analysis tasks: Insights from model midbrain responses. J Acoust Soc Am 147:3523–3537
Meaud J, Grosh K (2014) Effect of the attachment of the tectorial membrane on cochlear micromechanics and two-tone suppression. Biophys J 106:1398–1405
Meddis R, Õmard LP, Lopez-Poveda EA (2001) A computational algorithm for computing nonlinear auditory frequency selectivity. J Acoust Soc Am 109:2852–2861
Moleti A, Sisto R (2016) Localization of the reflection sources of stimulus-frequency otoacoustic emissions. J Assoc Res Otolaryngolol 17:393–401
Moleti A, Sisto R (2020) Suppression tuning curves in a two-degrees-of-freedom nonlinear cochlear model. J Acoust Soc Am 148:EL8–EL13
Moore BCJ (1978) Psychophysical tuning curves measured in simultaneous and forward masking. J Acoust Soc Am 63:524–532
Moore BCJ (1986) Parallels between frequency selectivity measured psychophysically and in cochlear mechanics. Scand Audiol Suppl 25:139–152
Moore BCJ, Glasberg BR, Roberts B (1984) Refining the measurement of psychophysical tuning curves. J Acoust Soc Am 76:1057–1066
Müller M, von Hünerbein K, Hoidis S, Smolders JW (2005) A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 202:63–73
Nam H, Guinan JJ (2018) Non-tip auditory-nerve responses that are suppressed by low-frequency bias tones originate from reticular lamina motion. Hear Res 358:1–9
Narayan SS, Temchin AN, Recio A, Ruggero MA (1998) Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science 282:1882–1884
Neely S (1980) Backward solution of a two-dimensional cochlear model. J Acoust Soc Am 67:S75–S75
Neely ST (1985) Mathematical modeling of cochlear mechanics. J Acoust Soc Am 78:345–352
Nobili R, Mammano F, Ashmore J (1998) How well do we understand the cochlea? Trends Neurosci 21:159–167
Oxenham AJ, Shera CA (2003) Estimates of human cochlear tuning at low levels using forward and simultaneous masking. J Assoc Res Otolaryngol 4:541–554
Pang XD, Guinan JJ (1997) Growth rate of simultaneous masking in cat auditory-nerve fibers: Relationship to the growth of basilar-membrane motion and the origin of two-tone suppression. J Acoust Soc Am 102:3564–3575
Patterson RD (1976) Auditory filter shapes derived with noise stimuli. J Acoust Soc Am 59:640–654
Patterson RD (1986) Auditory filters and excitation patterns as representations of frequency resolution. In: Moore BCJ (ed) Frequency Selectivity in Hearing. Academic, Berlin, pp 123–177
Peterson LC, Bogert BP (1950) A dynamical theory of the cochlea. J Acoust Soc Am 22:369–381
Rabbitt RD (2020) The cochlear outer hair cell speed paradox. Proc Natl Acad Sci USA 117:21880–21888
Rahman M, Willmore BDB, King AJ, Harper NS (2020) Simple transformations capture auditory input to cortex. Proc Natl Acad Sci USA 117:28442–28451
Rasetshwane DM, Bosen EC, Kopun JG, Neely ST (2019) Comparison of distortion-product otoacoustic emission and stimulus-frequency otoacoustic emission two-tone suppression in humans. J Acoust Soc Am 146:4481–4492
Rhode WS (1971) Observations of the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique. J Acoust Soc Am 49:1218–1231
Robles L, Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81:1305–1352
Ruggero MA, Temchin AN (2005) Unexceptional sharpness of frequency tuning in the human cochlea. Proc Natl Acad Sci USA 102:18614–18619
Ruggero MA, Robles L, Rich NC (1992) Two-tone suppression in the basilar membrane of the cochlea: Mechanical basis of auditory-nerve rate suppression. J Neurophysiol 68:1087–1099
Sachs MB, Kiang NY (1968) Two-tone inhibition in auditory-nerve fibers. J Acoust Soc Am 43(5):1120–1128
Scharf B (1959) Critical bands and the loudness of complex sounds near threshold. J Acoust Soc Am 31:365–370
Shera CA (2001) Frequency glides in click responses of the basilar membrane and auditory nerve: Their scaling behavior and origin in traveling-wave dispersion. J Acoust Soc Am 109:2023–2034
Shera CA (2001) Intensity-invariance of fine time structure in basilar-membrane click responses: Implications for cochlear mechanics. J Acoust Soc Am 110(1):332–348
Shera CA (2007) Laser amplification with a twist: Traveling-wave propagation and gain functions from throughout the cochlea. J Acoust Soc Am 122:2738–2758
Shera CA, Cooper NP (2013) Basilar-membrane interference patterns from multiple internal reflection of cochlear traveling waves. J Acoust Soc Am 133:2224–2239
Shera CA, Zweig G (1991) A symmetry suppresses the cochlear catastrophe. J Acoust Soc Am 89:1276–1289
Shera CA, Tubis A, Talmadge CL (2004a) Do forward-and backward-traveling waves occur within the cochlea? Countering the critique of Nobili et al. J Assoc Res Otolaryngol 5:349–359
Shera CA, Tubis A, Talmadge CL, Guinan JJ (2004) The dual effect of ‘suppressor’ tones on stimulus-frequency otoacoustic emissions. Assoc Res Otolaryngol Abstr 27:538
Shera CA, Tubis A, Talmadge CL (2005) Coherent reflection in a two-dimensional cochlea: Short-wave versus long-wave scattering in the generation of reflection-source otoacoustic emissions. J Acoust Soc Am 118:287–313
Shera CA, Guinan JJ, Oxenham AJ (2010) Otoacoustic estimation of cochlear tuning: Validation in the chinchilla. J Assoc Res Otolaryngol 11:343–365
Siebert WM (1974) Ranke revisited a simple short-wave cochlear model. J Acoust Soc Am 56:594–600
Sisto R, Moleti A (1999) Modeling otoacoustic emissions by active nonlinear oscillators. J Acoust Soc Am 106:1893–1906
Steele CR, Taber LA (1979) Comparison of WKB calculations and experimental results for three-dimensional cochlear models. J Acoust Soc Am 65:1007–1018
Stoop R, Kern A (2004) Two-tone suppression and combination tone generation as computations performed by the Hopf cochlea. Phys Rev Lett 93: 268103
Sumner CJ, Wells TT, Bergevin C, Sollini J, Kreft HA, Palmer AR, Oxenham AJ, Shera CA (2018) Mammalian behavior and physiology converge to confirm sharper cochlear tuning in humans. Proc Natl Acad Sci USA 115:11322–11326
Talmadge CL, Tubis A, Long GR, Tong C (2000) Modeling the combined effects of basilar membrane nonlinearity and roughness on stimulus frequency otoacoustic emission fine structure. J Acoust Soc Am 108:2911–2932
Temchin AN, Rich NC, Ruggero MA (2008) Threshold tuning curves of chinchilla auditory-nerve fibers. I. Dependence on characteristic frequency and relation to the magnitudes of cochlear vibrations. J Neurophysiol 100:2889–2898
Vavakou A, Cooper NP, van der Heijden M (2019) The frequency limit of outer hair cell motility measured in vivo. Elife 8: e47667
Vencovskỳ V, Vetešník A, Gummer AW (2020) Nonlinear reflection as a cause of the short-latency component in stimulus-frequency otoacoustic emissions simulated by the methods of compression and suppression. J Acoust Soc Am 147:3992–4008
Versteegh CP, van der Heijden M (2013) The spatial buildup of compression and suppression in the mammalian cochlea. J Assoc Res Otolaryngol 14:523–545
Wegel RL, Lane CE (1924) The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Phys Rev 23:266
Zhou W, Nam JH (2019) Probing hair cell’s mechano-transduction using two-tone suppression measurements. Sci Rep 9:1–16
Zilany MSA, Bruce IC (2006) Modeling auditory-nerve responses for high sound pressure levels in the normal and impaired auditory periphery. J Acoust Soc Am 120:1446–1466
Zweig G (1991) Finding the impedance of the organ of Corti. J Acoust Soc Am 89:1229–1254
Zweig G (2015) Linear cochlear mechanics. J Acoust Soc Am 138:1102–1121
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98:2018–2047
Zweig G, Lipes R, Pierce J (1976) The cochlear compromise. J Acoust Soc Am 59:975–982
Zwicker E (1961) Subdivision of the audible frequency range into critical bands (Frequenzgruppen). J Acoust Soc Am 33:248–248
Acknowledgements
We thank Michael G. Heinz, Enrique A. Lopez-Poveda, and two anonymous reviewers for their comments and constructive criticism. Supported by NIH/NIDCD grants R01 DC003687 (CAS), K99 DC016906-01A1(KKC), F32 DC016211 and R21 DC019209 (JBD), R01 DC017741 and R01 DC014450 (JSO), and INAIL grant BRIC 2019 ID-9 (RS).
Author information
Authors and Affiliations
Corresponding author
Appendices
APPENDIX A: APPROXIMATION OF SPATIAL GAIN FROM MEASURED BM TRANSFER FUNCTIONS
The assumption of local scaling symmetry means that BM transfer functions are identical near their peaks when the frequency axis is normalized to the local characteristic frequency (CF), that is, when frequencies are expressed as a function of the normalized variable \(\beta =f/\mathrm{CF}(\textit{x})\). In other words,
when
Scaling enables one to estimate the cochlear response to a specific frequency at any location by knowing (i) the BM transfer function at one location and (ii) the cochlear position-frequency map. Assuming that the tonotopic map is approximately exponential [\(\mathrm{CF}(\textit{x})=\mathrm{CF}(0)e^{-x/l}\), with l species dependent space constant] leads to
where \(\nu =\log _2{\beta }\) is the normalized frequency expressed in octaves.
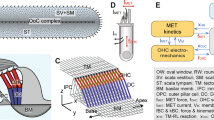
APPENDIX B: 3-D MODEL OF THE MOUSE COCHLEA
A) Longitudinal and B) cross-sectional geometry of the mouse model. (C) Acoustic area of the scalae of the mouse cochlea, extrapolated from Fig. 5 of Burda et al. (1988). The values are peak-normalized. The solid line represents the data, while the dotted line represents a curve with slope \(e^{-x/2l}\), where x is the distance from the cochlear base and l the space constant of the mouse frequency map. (D) Radius of the scalae, extrapolated by fitting a circle to the total cross-sectional area of the scalae (from Fig. 5 of Burda et al. 1988). The dotted line represents a linear fitting function \(h=0.13(1-x/L)+0.12\), with L the length of the cochlea (assumed here of 5.2 mm) used to compute the model’s solution. The fitting functions in panels C and D are used to compute the model’s solution [Eqs. (18,19)]
The model of the mouse cochlea used in this paper is based upon the work of Zweig (2015) and Altoè and Shera (2020a, 2020b). The model incorporates a highly simplified description of the cochlear 3-D hydrodynamics in conjunction with a scaling symmetric admittance of the cochlear partition (CP, the BM and organ of Corti). In this appendix, we present an alternate derivation of the WKB solution presented in Altoè and Shera (2020a), obtained using a different set of simplifying assumptions and geometry in order to highlight the generality of the model’s solution character.
Figure 6A, B presents the longitudinal and cross-sectional view of the model. The cochlear duct and the CP are assumed, for simplicity, of circular cross section of radii h and d respectively. The effective, acoustic width of the BM is \(b=2d\). For simplicity, the CP is assumed centered with respect to the scalae. Because we focus on the slow-propagating wave component—that represents the pressure difference across the cochlear partition and the motion of the CP center of mass—we neglect effects of possible CP deformations. That is, we consider the mode of vibration consisting of the CP moving up and down “en bloc”. A general solution for this model is given by de Boer (1980) in terms of Bessel functions.
In the mouse model, the cochlea is assumed 5.2 mm long, with an exponential location-frequency map
with space constant \(l=1.7\) mm and highest CF [\(\mathrm {CF}(0)\)] of 78 kHz, based upon Müller et al. (2005).
Assuming harmonic time dependence, the relationship between pressure difference across the organ of Corti (\(P_d\)) and BM velocity (\(V_{\mathrm {BM}}\)) is expressed through an effective acoustic admittance
which is a function of location x and angular frequency \(\omega\). Since the cochlea is nonlinear, \(Y_{\mathrm {CP}}\) represents a sinusoidal describing function. For notational simplicity, dependencies on frequency and location are left implicit in what follows.
The longitudinal fluid flow in the cochlea can be conveniently described by standard transmission-line theory (Shera et al. 2005). This requires introducing the variable \(\bar{P}\) representing the pressure difference across the scalae averaged over their cross-sectional area (Duifhuis 1988). The function \(\alpha =P_d/\bar{P}\) quantifies the ratio between driving and average pressure. With this stratagem, Newton’s second law and mass conservation imply that
respectively. In these equations U represents the (longitudinal) volume velocity, \(\rho\) the density of the fluids, b the width of the BM, and S the acoustic area of the scalae [\(S=S_{\mathrm{st}}S_{\mathrm{sv}}/(S_{\mathrm{st}}+S_{\mathrm{sv}}\))]. Combining Eqs. (14-15) leads to the Webster horn equation familiar from acoustics (Peterson and Bogert 1950)
where k, the complex wavenumber, is (Duifhuis 1988)
The WKB solution of Eq. (16) is (Altoè and Shera 2020a)
where \(P_0\) is the pressure at the cochlear entrance. Due to the radial symmetry of the model, the 3-D pressure field [P(x, y, z)] is conveniently described in cylindrical coordinates as a function of location x and radial position [distance \(r'\) from the CP center and angle \(\phi\) : \(P(x,r',\phi )\)]. When the CP is small enough, gradients of the pressure field along \(\phi\) can be regarded to play a secondary role for the model’s solution (see also de Boer 1980, 1981), and hence the 3-D field is, for simplicity, approximated by a 2-D field [\(P(x,r',\phi )\approx P(x,r')\)]. With these simplification we can adopt the 2-D approximation for \(\alpha\) given by Duifhuis (1988)
The model solution depends on how S and h vary along the cochlea; their values for the mouse cochlea are obtained by fitting simple functions to published morphometric data (Burda et al. 1988) in Fig. 6C, D. Although the pressure field is nearly 2-D, the model effectively has three spatial dimensions because the BM width is shorter than the scalae radius [Eq. 15]. The narrow BM produces large differences between 2- and 3-D models (de Boer 1981; Zweig 2015). Alternative and more general solutions to 3-D models fundamentally rely on describing the pressure field as a superposition of distinct modes that satisfy the boundary condition at the wall separating the two scalae (see, e.g., Steele and Taber 1979; Lighthill 1981; de Boer 1981).
The mechanical admittance of the CP is taken to be (Zweig 2015; Altoè and Shera 2020b)
where \(\beta =f/\mathrm {CF}\) is the ratio between frequency and characteristic frequency (\(\mathrm {CF}\)) at the specific location, m is the acoustic mass of the CP, \(\zeta\) is the damping coefficient, and \(\tau\) is a level-dependent parameter that controls the magnitude of the active term, \(i\tau \beta\), which represents an active pressure proportional to the time-derivative of the driving pressure. The value of \(\tau\) at each cochlear location is determined by
where \(\tau _0\) is the coefficient of the active force term at the lowest sound levels, and \(P_{\mathrm{sat}}\) a constant, independent of location, that controls the level-dependence of \(\tau\). For simulations using two tones of normalized frequencies \(\beta _1\) and \(\beta _2\), eliciting pressures \(P_1\) and \(P_2\) respectively, the value of \(\tau\) is calculated at each location as
The model solution is determined iteratively starting from a linear solution consisting of the linear superposition of the responses to the two tones separately. The value of \(\tau\) at each cochlear location is then computed and used to update the values of k and \(\alpha\). This procedure is iterated 10 times; within each step of the solution, the values of k and \(\alpha\) are determined by iterating Eqs. (17) and (19) (also 10 times).
APPENDIX C: LOW- AND HIGH-SIDE SUPPRESSION
This appendix elucidates the differences between high-side and low-side suppression using the simple traveling-wave framework. The explanation is conceptually equivalent to that given by Versteegh and van der Heijden (2013). Cooper (1996) and Versteegh and van der Heijden (2013) noted that both above-CF and below-CF suppressors can greatly suppress the BM response to a near-CF tone. However, the total BM response (i.e., the superposition of suppressor and probe response) increases with suppressor level when using below-CF suppressors (low-side suppression) whereas it decreases when using above-CF suppressors (high-side suppression). In light of the simple traveling-wave framework, the explanation for this difference is straightforward. Consider, for example, the case when the suppressor frequency is well below CF. The BM response to this low-frequency suppressor will be nearly linear because at tail frequencies the BM wave undergoes little spatial amplification. Nonetheless, this suppressor, by exciting the BM in the active region where a near-CF probe tone is spatially amplified, will activate the cochlear nonlinearity and hence attenuate the BM probe response. As a result, the probe response is attenuated, but not the suppressor response. Therefore, the overall BM response will increase with suppressor level, despite the significant suppressive effects at play.
Conversely, above-CF suppressors will attenuate the near-CF probe without eliciting a significant response on the BM (phantom suppression). As a consequence, increasing the level of above-CF suppressors will reduce the overall BM response, as this is dominated by the probe response. Figure 7A,B shows the model’s two-tone responses as a function of suppressor level, showing that the model captures the differences between low- and high-side suppression via the mechanisms of nonlinear wave amplification elucidated throughout this study. Note that accounting for differences between low- and high-side suppression is particularly straightforward using the traveling-wave framework because the activation of the cochlear nonlinearity is rather frequency-independent—it depends on local excitation level, without additional “filtering”—whereas its effects (i.e., the reduction of spatial amplification) are strongly frequency dependent (e.g., they affect the response to near-CF but not tail frequency tones). This dichotomy between the causes and the effects of cochlear nonlinearity is readily observed in the experimental data (e.g., Dong and Olson 2013; Vavakou et al. 2019; Fallah et al. 2019; Dewey et al. 2019), and naturally emerges from the long- and short-wave hydrodynamics at tail and peak frequencies, respectively (Altoè and Shera 2020b).
BM model responses to suppressor and probe tones as a function of suppressor level. The probe is a 40 dB CF-tone (9.3 kHz), while the suppressor level is indicated in the abscissa. The colored lines indicate results obtained using suppressors of different frequencies. A) Overall BM response magnitude, i.e., sum of magnitudes of suppressor and probe frequency components. B) BM magnitude response at the probe frequency. Panel B highlights that a near-CF probe response monotonically decreases with increasing suppressor level—regardless of suppressor frequency. Panel A shows that the overall excitation level of the BM either decreases or increases with suppressor level depending on whether the suppressor frequency is above or below CF, respectively.
Rights and permissions
About this article
Cite this article
Altoè, A., Charaziak, K.K., Dewey, J.B. et al. The Elusive Cochlear Filter: Wave Origin of Cochlear Cross-Frequency Masking. JARO 22, 623–640 (2021). https://doi.org/10.1007/s10162-021-00814-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-021-00814-2