ABSTRACT
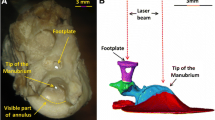
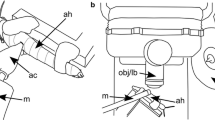
The vibratory responses to tones of the stapes and incus were measured in the middle ears of deeply anesthetized chinchillas using a wide-band acoustic-stimulus system and a laser velocimeter coupled to a microscope. With the laser beam at an angle of about 40 ° relative to the axis of stapes piston-like motion, the sensitivity-vs.-frequency curves of vibrations at the head of the stapes and the incus lenticular process were very similar to each other but larger, in the range 15–30 kHz, than the vibrations of the incus just peripheral to the pedicle. With the laser beam aligned with the axis of piston-like stapes motion, vibrations of the incus just peripheral to its pedicle were very similar to the vibrations of the lenticular process or the stapes head measured at the 40 ° angle. Thus, the pedicle prevents transmission to the stapes of components of incus vibration not aligned with the axis of stapes piston-like motion. The mean magnitude curve of stapes velocities is fairly flat over a wide frequency range, with a mean value of about 0.19 mm.(s Pa−1), has a high-frequency cutoff of 25 kHz (measured at −3 dB re the mean value), and decreases with a slope of about −60 dB/octave at higher frequencies. According to our measurements, the chinchilla middle ear transmits acoustic signals into the cochlea at frequencies exceeding both the bandwidth of responses of auditory-nerve fibers and the upper cutoff of hearing. The phase lags of stapes velocity relative to ear-canal pressure increase approximately linearly, with slopes equivalent to pure delays of about 57–76 μs.








Similar content being viewed by others
REFERENCES
Bode HW (1945) Network analysis and feedback amplifier design. D. Van Nostrand Company, Inc, New York
Chan JC, Musicant AD, Hind JE (1993) An insert earphone system for delivery of spectrally shaped signals for physiological studies. J Acoust Soc Am 93:1496–1501
Décory L, Franke RB, Dancer AL (1990) Measurement of the middle ear transfer function in cat, chinchilla and guinea pig. In: The Mechanics and Biophysics of Hearing (Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, eds), pp 270-277. Berlin: Springer.
de la Rochefoucauld O, Decraemer WF, Khanna SM, Olson ES (2008) Simultaneous measurements of ossicular velocity and intracochlear pressure leading to the cochlear input impedance in gerbil. J Assoc Res Otolaryngol 9:161–177
de la Rochefoucauld O, Kachroo P, Olson ES (2010) Ossicular motion related to middle ear transmission delay in gerbil. Hear Res 270:158–172
Decraemer WF, de la Rochefoucauld O, Dong W, Khanna SM, Dirckx JJ, Olson ES (2007a) Scala vestibuli pressure and three-dimensional stapes velocity measured in direct succession in gerbil. J Acoust Soc Am 121:2774–2791
Decraemer WF, de la Rochefoucauld O, Khanna SM, Dirckx JJJ (2007b) The pedicle of lenticular process in gerbil, cat and human does not produce a boost at the high frequency limit of the audible range. Assoc Res Otolaryngol Midwinter Meet Abstr 30:182
Edom E, Obrist D, Henniger R, Kleiser L, Sim JH, Huber AM (2013) The effect of rocking stapes motions on the cochlear fluid flow and on the basilar membrane motion. J Acoust Soc Am 134:3749–3758
Elkhouri N, Liu H, Funnell WR (2006) Low-frequency finite-element modeling of the gerbil middle ear. J Assoc Res Otolaryngol JARO 7:399–411
Fay RR (1988) Hearing in vertebrates: a psychophysics databook. Hill-Fay Associates, Winnetka
Fay JP, Puria S, Steele CR (2006) The discordant eardrum. Proc Natl Acad Sci U S A 103:19743–19748
Funnell WR, Siah TH, McKee MD, Daniel SJ, Decraemer WF (2005) On the coupling between the incus and the stapes in the cat. J Assoc Res Otolaryngol JARO 6:9–18
Heffner RS, Heffner HE (1991) Behavioral hearing range of the chinchilla. Hear Res 52:13–16
Javel E, McGee JA, Horst JW, Farley GR (1988) Temporal mechanisms in auditory stimulus coding. In: Edelman GM, Gall WE, Cowan WM (eds) Auditory function—neurobiological bases of hearing. Wiley, New York, pp 515–548
Johnson DH (1980) The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am 68:1115–1122
Koka K, Holland NJ, Lupo JE, Jenkins HA, Tollin DJ (2010) Electrocochleographic and mechanical assessment of round window stimulation with an active middle ear prosthesis. Hear Res 263:128–137
Miller J (1970) Audibility curve of the chinchilla. J Acoust Soc Am 48:513–523
Murphy WJ, Davis RR (1998) The role of the chinchilla pinna and ear canal in electrophysiological measures of hearing thresholds. J Acoust Soc Am 103:1951–1956
Narayan SS, Temchin AN, Recio A, Ruggero MA (1998) Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science 282:1882–1884
Olson ES (1998) Observing middle and inner ear mechanics with novel intracochlear pressure sensors. J Acoust Soc Am 103:3445–3463
Overstreet EH, Ruggero MA (2002) Development of wide-band middle ear transmission in the Mongolian gerbil. J Acoust Soc Am 111:261–270
Puria S, Allen JB (1998) Measurements and model of the cat middle ear: evidence of tympanic membrane acoustic delay. J Acoust Soc Am 104:3463–3481
Ravicz ME, Rosowski JJ (2012) Chinchilla middle-ear admittance and sound power: high-frequency estimates and effects of inner-ear modifications. J Acoust Soc Am 132:2437–2454
Ravicz ME, Rosowski JJ (2013a) Inner-ear sound pressures near the base of the cochlea in chinchilla: further investigation. J Acoust Soc Am 133:2208–2223
Ravicz ME, Rosowski JJ (2013b) Middle-ear velocity transfer function, cochlear input immittance, and middle-ear efficiency in chinchilla. J Acoust Soc Am 134:2852–2865
Ravicz ME, Olson ES, Rosowski JJ (2007) Sound pressure distribution and power flow within the gerbil ear canal from 100 Hz to 80 kHz. J Acoust Soc Am 122:2154–2173
Ravicz ME, Cooper NP, Rosowski JJ (2008) Gerbil middle-ear sound transmission from 100 Hz to 60 kHz. J Acoust Soc Am 124:363–380
Ravicz ME, Slama MC, Rosowski JJ (2010) Middle-ear pressure gain and cochlear partition differential pressure in chinchilla. Hear Res 263:16–25
Robles L, Temchin AN, Fan Y-H, Ruggero MA (2005) Boost of transmission at the incudo-stapedial joint of chinchilla middle ear. Assoc Res Otolaryngol Midwinter Meet Abstr 28:321–322
Robles L, Temchin AN, Fan Y-H, Cai H, Ruggero MA (2006) Vibrations of the stapes and the long and lenticular processes of the incus in the chinchilla middle ear. Assoc Res Otolaryngol Midwinter Meet Abstr 29:215
Rosowski JJ, Ravicz ME, Songer JE (2006) Structures that contribute to middle-ear admittance in chinchilla. J Comp Physiol Neuroethol Sens Neural Behav Physiol 192:1287–1311
Ruggero MA, Temchin AN (2002) The roles of the external, middle, and inner ears in determining the bandwidth of hearing. Proc Natl Acad Sci U S A 99:13206–13210
Ruggero MA, Rich NC, Robles L, Shivapuja BG (1990) Middle-ear response in the chinchilla and its relationship to mechanics at the base of the cochlea. J Acoust Soc Am 87:1612–1629
Ruggero MA, Narayan SS, Temchin AN, Recio A (2000) Mechanical bases of frequency tuning and neural excitation at the base of the cochlea: comparison of basilar-membrane vibrations and auditory-nerve-fiber responses in chinchilla. Proc Natl Acad Sci U S A 97:11744–11750
Ruggero MA, Temchin AN, Robles L, Overstreet EH (2004) A new and improved middle ear. Proceedings of the 3rd Symposium on Middle Ear Mechanics in Research and Otology 134–141
Ruggero MA, Temchin AN, Fan YH, Cai HX, Robles L (2007) Boost of Transmission at the Pedicle of the Incus in the Chinchilla Middle Ear. In: Middle Ear Mechanics in Research and Otology (Huber A, Eiber A, eds), pp 154-157. Singapore: World Scientific.
Slama MC, Ravicz ME, Rosowski JJ (2010) Middle ear function and cochlear input impedance in chinchilla. J Acoust Soc Am 127:1397–1410
Songer JE, Rosowski JJ (2006) The effect of superior-canal opening on middle-ear input admittance and air-conducted stapes velocity in chinchilla. J Acoust Soc Am 120:258–269
Songer JE, Rosowski JJ (2007) Transmission matrix analysis of the chinchilla middle ear. J Acoust Soc Am 122:932–942
Temchin AN, Ruggero MA (2010) Phase-locked responses to tones of chinchilla auditory nerve fibers: implications for apical cochlear mechanics. Jaro J Assoc Res Otolaryngol 11:297–318
Temchin AN, Robles L, Ruggero MA (2002) A re-examination of middle-ear transmission in chinchilla. Assoc Res Otolaryngol Midwinter Meet Abstr 25:154
Temchin AN, Rich NC, Ruggero MA (2008a) Threshold tuning curves of chinchilla auditory-nerve fibers. I. Dependence on characteristic frequency and relation to the magnitudes of cochlear vibrations. J Neurophysiol 100:2889–2898
Temchin AN, Rich NC, Ruggero MA (2008b) Threshold tuning curves of chinchilla auditory nerve fibers. II. Dependence on spontaneous activity and relation to cochlear nonlinearity. J Neurophysiol 100:2899–2906
von Bismarck G (1967) The sound pressure transformation function from free-field to the eardrum of chinchilla. (Master's Dissertation, Dept. of Electrical Engineering, Massachusetts Institute of Technology), pp 1-93. Cambridge, MA.
Wilson JP, Bruns V (1983) Middle-ear mechanics in the CF-bat Rhinolophus ferrumequinum. Hear Res 10:1–13
ACKNOWLEDGMENTS
This work was supported by Grant DC-000419 from the National Institute on Deafness and Other Communication Disorders. L.R. was partially funded by Grant Fondecyt 1120256 (Chile).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robles, L., Temchin, A.N., Fan, YH. et al. Stapes Vibration in the Chinchilla Middle Ear: Relation to Behavioral and Auditory-Nerve Thresholds. JARO 16, 447–457 (2015). https://doi.org/10.1007/s10162-015-0524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-015-0524-x