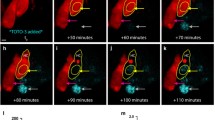
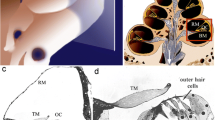
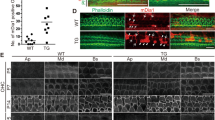
Abstract
Hair cell death is a major cause of hearing impairment. Preservation of surface barrier upon hair cell loss is critical to prevent leakage of potassium-rich endolymph into the organ of Corti and to prevent expansion of cellular damage. Understanding of wound healing in this cytoarchitecturally complex organ requires ultrastructural 3D visualization. Powered by the serial block-face scanning electron microscopy, we penetrate into the cell biological mechanisms in the acute response of outer hair cells and glial-like Deiters’ cells to ototoxic trauma in vivo. We show that Deiters’ cells function as phagocytes. Upon trauma, their phalangeal processes swell and the resulting close cellular contacts allow engulfment of apoptotic cell debris. Apical domains of dying hair cells are eliminated from the inner ear sensory epithelia, an event thought to depend on supporting cells’ actomyosin contractile activity. We show that in the case of apoptotic outer hair cells of the organ of Corti, elimination of their apices is preceded by strong cell body shrinkage, emphasizing the role of the dying cell itself in the cleavage. Our data reveal that the resealing of epithelial surface by junctional extensions of Deiters’ cells is dynamically reinforced by newly polymerized F-actin belts. By analyzing Cdc42-inactivated Deiters’ cells with defects in actin dynamics and surface closure, we show that compromised barrier integrity shifts hair cell death from apoptosis to necrosis and leads to expanded hair cell and nerve fiber damage. Our results have implications concerning therapeutic protective and regenerative interventions, because both interventions should maintain barrier integrity.









Similar content being viewed by others
References
Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y (2006) The fate of outer hair cells after acoustic or ototoxic insults. Hear Res 218:20–9
Ahmad M, Bohne BA, Harding GW (2003) An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res 175:82–100
Anttonen T, Kirjavainen A, Belevich I, Laos M, Richardson WD, Jokitalo E, Brakebusch C, Pirvola U (2012) Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci Rep 2:978
Bird JE, Daudet N, Warchol ME, Gale JE (2010) Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J Neurosci 30:12545–56
Bohne BA, Harding GW, Lee SC (2007) Death pathways in noise-damaged outer hair cells. Hear Res 223:61–70
Burns JC, Corwin JT (2013) A historical to present-day account of efforts to answer the question: “what puts the brakes on mammalian hair cell regeneration?”. Hear Res 297:52–67
Burns JC, Corwin JT (2014) Responses to cell loss become restricted as the supporting cells in mammalian vestibular organs grow thick junctional actin bands that develop high stability. J Neurosci 34:1998–2011
Burns JC, Collado MS, Oliver ER, Corwin JT (2013) Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. J Comp Neurol 521:1430–48
Cotanche DA, Dopyera CE (1990) Hair cell and supporting cell response to acoustic trauma in the chick cochlea. Hear Res 46:29–40
Crouch JJ, Sakaguchi N, Lytle C, Schulte BA (1997) Immunohistochemical localization of the Na–K–Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem 45:773–8
Denk W, Horstmann H (2004) Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2:e329
Flock A, Flock B, Fridberger A, Scarfone E, Ulfendahl M (1999) Supporting cells contribute to control of hearing sensitivity. J Neurosci 19:4498–507
Forge A (1985) Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res 19:171–82
Forge A, Jagger DJ, Kelly JJ, Taylor RR (2013) Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J Cell Sci 126:1703–12
Fredelius L (1988) Time sequence of degeneration pattern of the organ of corti after acoustic overstimulation. A transmission electron microscopy study. Acta Otolaryngol 106:373–85
Fuchs PA, Lehar M, Hiel H (2014) Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol 522:717–29
Gale JE, Meyers JR, Periasamy A, Corwin JT (2002) Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog’s saccule. J Neurobiol 50:81–92
Guillot C, Lecuit T (2013) Mechanics of epithelial tissue homeostasis and morphogenesis. Science 340:1185–9
Hirose K, Westrum LE, Cunningham DE, Rubel EW (2004) Electron microscopy of degenerative changes in the chick basilar papilla after gentamicin exposure. J Comp Neurol 470:164–80
Hochreiter-Hufford A, Ravichandran KS (2013) Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 5:a008748
Hordichok AJ, Steyger PS (2007) Closure of supporting cell scar formations requires dynamic actin mechanisms. Hear Res 232:1–19
Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, Gale JE (2014) Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J Cell Sci 127:1229–41
Kurihara K, Moore-Hoon ML, Saitoh M, Turner RJ (1999) Characterization of a phosphorylation event resulting in upregulation of the salivary Na(+)–K(+)–2Cl(−) cotransporter. Am J Physiol 277:1184–C1193
Leonova EV, Raphael Y (1997) Organization of cell junctions and cytoskeleton in the reticular lamina in normal and ototoxically damaged organ of corti. Hear Res 113:14–28
MacVicar BA, Feighan D, Brown A, Ransom B (2002) Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia 37:114–23
McDowell B, Davies S, Forge A (1989) The effect of gentamicin-induced hair cell loss on the tight junctions of the reticular lamina. Hear Res 40:221–32
Meyers JR, Corwin JT (2007) Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci 27:4313–25
Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA (2005) Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 12:107–14
Monzack EL, Cunningham LL (2013) Lead roles for supporting actors: critical functions of inner ear supporting cells. Hear Res 303:20–9
Oesterle EC (2013) Changes in the adult vertebrate auditory sensory epithelium after trauma. Hear Res 297:91–8
Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR (2008) Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol 9:65–89
Parnaik R, Raff MC, Scholes J (2000) Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol 10:857–60
Piazza V, Ciubotaru CD, Gale JE, Mammano F (2007) Purinergic signalling and intercellular Ca2+ wave propagation in the organ of corti. Cell Calcium 41:77–86
Raphael Y, Altschuler RA (1991) Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton 18:215–27
Raphael Y, Altschuler RA (2003) Structure and innervation of the cochlea. Brain Res Bull 60:397–422
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16:522–9
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11:1847–57
Sakaguchi N, Crouch JJ, Lytle C, Schulte BA (1998) Na–K–Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res 118:114–22
Saunders JC, Dear SP, Schneider ME (1985) The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am 78:833–60
Silva MT (2010) Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett 584:4491–9
Sonnemann KJ, Bement WM (2011) Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu Rev Cell Dev Biol 27:237–63
Souter M, Nevill G, Forge A (1997) Postnatal maturation of the organ of corti in gerbils: morphology and physiological responses. J Comp Neurol 386:635–51
Taylor RR, Nevill G, Forge A (2008) Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol 9:44–64
Taylor RR, Jagger DJ, Forge A (2012) Defining the cellular environment in the organ of corti following extensive hair cell loss: a basis for future sensory cell replacement in the cochlea. PLoS One 7:e30577
Wang F, Wang F, Zou Z, Liu D, Wang J, Su Y (2011) Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Lab Invest 91:462–71
Wilke SA, Antonios JK, Bushong EA, Badkoobehi A, Malek E, Hwang M, Terada M, Ellisman MH, Ghosh A (2013) Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J Neurosci 33:507–22
Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, Brakebusch C (2006) Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn 236:2767–78
Ylikoski J (1974) Correlative studies on the cochlear pathology and hearing loss in guinea-pigs after intoxication with ototoxic antibiotics. Acta Otolaryngol Suppl 326:1–62
Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD (2010) An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia 58:943–53
Acknowledgments
This work was supported by the Academy of Finland, Jane and Aatos Erkko Foundation, Instrumentarium Foundation (U.P.), and Biocenter Finland. The authors thank Sanna Sihvo and Antti Salminen for excellent technical assistance, and R.J. Turner and K. Kaila for the generous gift of the NKCC1 antibody.
Conflict of Interest
The authors declare that they have no conflicts of interest, financial or otherwise, regarding this work.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Movie of 3D reconstructed DCs and OHCs at E18, demonstrating spatial relationship between these cell types. Nonlesioned cochlea. (MP4 5437 kb)
Movie of 3D reconstructed DCs and OHCs at P10, demonstrating spatial relationship between these cell types. Nonlesioned cochlea. (MP4 5329 kb)
Movie scanning up and down through serial sections, showing phagocytotic OHC debris within the phalangeal process of a DC. Movie starts from the reticular lamina and extends to the base of the phalangeal process. Acute lesion site. (MP4 4545 kb)
Movie of the 3D reconstructed bundleless OHC. Mitochondria, nucleus, innervated nerve fibers and the contour of the cell have been reconstructed. Late lesion site. (MP4 5234 kb)
Movie scanning up and down through serial sections from the late lesion site, showing 3D reconstruction of a DC cup, the OHC base within the cup and innervated nerve fibers. Nonlesioned cochlea. (MP4 7777 kb)
Rights and permissions
About this article
Cite this article
Anttonen, T., Belevich, I., Kirjavainen, A. et al. How to Bury the Dead: Elimination of Apoptotic Hair Cells from the Hearing Organ of the Mouse. JARO 15, 975–992 (2014). https://doi.org/10.1007/s10162-014-0480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-014-0480-x