Abstract
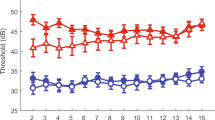
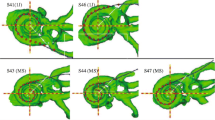
This study examines the relationship between focused-stimulation thresholds, electrode positions, and speech understanding in deaf subjects treated with a cochlear implant (CI). Focused stimulation is more selective than monopolar stimulation, which excites broad regions of the cochlea, so may be more sensitive as a probe of neural survival patterns. Focused thresholds are on average higher and more variable across electrodes than monopolar thresholds. We presume that relatively high focused thresholds are the result of larger distances between the electrodes and the neurons. Two factors are likely to contribute to this distance: (1) the physical position of electrodes relative to the modiolus, where the excitable auditory neurons are normally located, and (2) the pattern of neural survival along the length of the cochlea, since local holes in the neural population will increase the distance between an electrode and the nearest neurons. Electrode-to-modiolus distance was measured from high-resolution CT scans of the cochleae of CI users whose focused-stimulation thresholds were also measured. A hierarchical set of linear models of electrode-to-modiolus distance versus threshold showed a significant increase in threshold with electrode-to-modiolus distance (average slope = 11 dB/mm). The residual of these models was hypothesized to reflect neural survival in each subject. Consonant–Nucleus–Consonant (CNC) word scores were significantly correlated with the within-subject variance of threshold (r 2 = 0.82), but not with within-subject variance of electrode distance (r 2 = 0.03). Speech understanding also significantly correlated with how well distance explained each subject’s threshold data (r 2 = 0.63). That is, subjects with focused thresholds that were well described by electrode position had better speech scores. Our results suggest that speech understanding is highly impacted by individual patterns of neural survival and that these patterns manifest themselves in how well (or poorly) electrode position predicts focused thresholds.






Similar content being viewed by others
References
Bierer JA (2007) Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am 121:1642.
Bierer JA (2010) Probing the electrode–neuron interface with focused cochlear implant stimulation. Trends Amplif 14:84–95.
Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, Larky J, Lindström B, Nedzelski J, Peterson A, Shipp D, Staller S, Whitford L (1996) Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol 1:293–306
Blamey PJ, Pyman BC, Gordon M, Clark GM, Brown AM, Dowell RC, Hollow RD (1992) Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol 101:342–8
Cohen LT, Saunders E, Clark GM (2001) Psychophysics of a prototype peri-modiolar cochlear implant electrode array. Hear Res 155:63–81
Fayad JN, Linthicum FH (2006) Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope 116:1310–20.
Garadat SN, Zwolan TA, Pfingst BE (2012) Across-site patterns of modulation detection: relation to speech recognition. J Acoust Soc Am 131:4030–41.
Gordon KA, Papsin BC (2013) From nucleus 24 to 513: changing cochlear implant design affects auditory response thresholds. Otol Neurotol 34:436–42.
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, Skinner MW (2013) Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 34:342–60.
Van den Honert C, Carlyon R, Long C, Smith Z, Shelton C, Kelsall D (2007) Perceptual evidence of spatially-restricted excitation with focused stimulation. Conf Implantable Audit Prostheses 2:58
Van den Honert C, Kelsall DC (2007) Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am 121:3703–16.
Judd CM, McClelland GH, Ryan CS (2009) Data analysis: a model comparison approach, 2nd ed. Routledge, New York, p 328
Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH (1998) Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol (Stockh) 118:313–326
Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB (2005) Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope 115:672–7.
Landry TG, Fallon JB, Wise AK, Shepherd RK (2013) Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity ofcochlear implants. J Comp Neurol. doi:10.1002/cne.23318
Leake PA, Stakhovskaya O, Hetherington A, Rebscher SJ, Bonham B (2013) Effects of brain-derived neurotrophic factor (BDNF) and electrical stimulation on survival and function of cochlear spiral ganglion neurons in deafened, developing cats. J Assoc Res Otolaryngol 14:187–211.
Levitt H (1971) Transformed up-down methods in psychophysics. J Acoust Soc Am 49:467–477
Li PM, Somdas MA, Eddington DK, Nadol JB (2007) Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol 116:731–8
Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN (2011) Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol 12:711–7.
Nadol JB, Young YS, Glynn RJ (1989) Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol 98:411–6
Nadol JB, Shiao J, Burgess B, Ketten D, Eddington D, Gantz B, Kos I, Montandon P, Coker N, Roland JJ, Shallop J (2001) Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 110:883–891
Noble JH, Labadie RF, Gifford R, Dawant B (2013) Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Trans Neural Syst Rehabil Eng. doi:10.1109/TNSRE.2013.2253333
Peterson GE, Lehiste I (1962) Revised CNC lists for auditory tests. J Speech Hear Dis 27:62–70
Pfingst BE, Xu L (2004) Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol 5:11–24.
Pfingst BE, Xu L, Thompson CS (2004) Across-site threshold variation in cochlear implants: relation to speech recognition. Audiol Neurootol 9:341–52.
Robb RA (2001) The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging 20:854–67.
Roland JT (2005) A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope 115:1325–39.
Roland P, Wright C (2006) Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol 64:11–30
Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxon EA, Hullar TE, Finley CC (2007) In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl 197:2–24
Smith ZM, Parkinson WS, Long CJ (2013) Multipolar current focusing increases spectral resolution in cochlear implants. Conf Proc IEEE Eng Med Biol Soc Jul 2013:2796–2799.
Stakhovskaya O, Sridhar D, Bonham BH, Leake PA (2007) Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol 8:220–33.
Teymouri J, Hullar TE, Holden TA, Chole RA (2011) Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otol Neurotol 32:980–6.
Todt I, Basta D, Eisenschenk A, Ernst A (2005) The “pull-back” technique for Nucleus 24 perimodiolar electrode insertion. Otolaryngol Head Neck Surg 132:751–4.
Vanpoucke F, Boermans P, Frijns J (2012) Assessing the placement of a cochlear electrode array by multidimensional scaling. IEEE Trans Biomed Eng 59:307–310
Voie AH (2002) Imaging the intact guinea pig tympanic bulla by orthogonal-plane fluorescence optical sectioning microscopy. Hear Res 171:119–128
Zwolan TA, Collins LM, Wakefield GH (1997) Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am 102:3673–85
Acknowledgments
CJL, ZMS, WSP are employed by Cochlear Ltd. We thank the patients for their hard work, the audiologists at the clinical study sites for programming and data collection, Chris van den Honert for numerous contributions, and two anonymous reviewers for their useful suggestions. TAH acknowledges the support of the National Institutes of Health, National Institute on Deafness and Communication Disorders R01 DC00581 and R01 DC009010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Long, C.J., Holden, T.A., McClelland, G.H. et al. Examining the Electro-Neural Interface of Cochlear Implant Users Using Psychophysics, CT Scans, and Speech Understanding. JARO 15, 293–304 (2014). https://doi.org/10.1007/s10162-013-0437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-013-0437-5