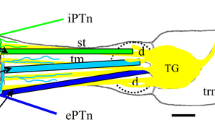
Abstract
Recently, three novel flexor muscles (M1, M2 and M3) in the posterior tentacles of the snail have been described, which are responsible for the patterned movements of the tentacles of the snail, Helix pomatia. In this study, we have demonstrated that the muscles received a complex innervation pattern via the peritentacular and olfactory nerves originating from different clusters of motoneurons of the cerebral ganglia. The innervating axons displayed a number of varicosities and established neuromuscular contacts of different ultrastructural forms. Contractions evoked by nerve stimulation could be mimicked by external acetylcholine (ACh) and glutamate (Glu), suggesting that ACh and Glu are excitatory transmitters at the neuromuscular contacts. Choline acetyltransferase and vesicular glutamate transporter immunolabeled axons innervating flexor muscles were demonstrated by immunohistochemistry and in Western blot experiments. Nerve- and transmitter-evoked contractions were similarly attenuated by cholinergic and glutamatergic antagonists supporting the dual excitatory innervation. Dopamine (DA, 10−5 M) oppositely modulated thin (M1/M2) and thick (M3) muscle responses evoked by stimulation of the olfactory nerve, decreasing the contractions of the M1/M2 and increasing those of M3. In both cases, the modulation site was presynaptic. Serotonin (5-HT) at high concentration (10−5 M) increased the amplitude of both the nerve- and the ACh-evoked contractions in all muscles. The relaxation rate was facilitated suggesting pre- and postsynaptic site of action. Our data provided evidence for a DAergic and 5-HTergic modulation of cholinergic nerves innervating flexor muscles of the tentacles as well as the muscles itself. These effects of DA and 5-HT may contribute to the regulation of sophisticated movements of tentacle muscles lacking inhibitory innervation.








Similar content being viewed by others
References
Calabrese RL (1989) Modulation of muscle and neuromuscular junctions in invertebrates. Semin Neurosci 1:25–34
Charnetski M, Palta M, Sahu R (2000) Dopaminergic modulation of excitatory junction potentials at the crayfish neuromuscular junction. Pioneer Neurosci 1:49–55
Chase R (2002) Behavior and its neural control in gastropod mollusks. Oxford University Press, Oxford
Chase R, Croll R (1981) Tentacular function in snail olfactory orientation. J Comp Physiol A 143:357–362
Chase R, Pryer K, Baker R, Medison D (1978) Responses to conspecific chemical stimuli in the terrestrial snail Achatina fulica. Behav Biol 22:302–315
Elekes K (2000) Ultrastructural aspects of peptidergic modulation in the peripheral nervous system of Helix pomatia. Microsc Res Tech 49(6):534–546
Elekes K, Ude J (1994) Peripheral connections of FMRFamide-like immunoreactive neurons in the snail, Helix pomatia: an immunogold electron microscopic study. J Neurocytol 23(12):758–769
Fox LE, Lloyd PE (1997) Serotonin and the small cardioactive peptides differentially modulate two motor neurons that innervate the same muscle fibers in Aplysia. J Neurosci 17(16):6064–6074
Friedrich A, Teyke T (1998) Identification of stimuli and input pathways mediating food-attraction conditioning in the snail, Helix. J Comp Physiol 183:247–254
Gelperin A (1974) Olfactory basis of homing behavior in the giant garden slug, Limax maximus. Proc Nat Acad Sci USA 71:966–970
Hernádi L, Teyke T (2012) Novel triplet of flexor muscles in the posterior tentacles of the snail, Helix pomatia. Acta Biol Hung 63(Suppl 2):123–128
Hernádi L, Teyke T (2013) Neuronal background of positioning of the posterior tentacles in the snail Helix pomatia. Cell Tissue Res 352:217–225
Heyer CB, Kater SB (1973) Neuromuscular system in molluscs. Am Zool 13:247–270
Hurwitz I, Cropper EC, Vilim FS, Alexeeva V, Susswein AJ, Kupfermann I et al (2000) Serotonergic and peptidergic modulation of the buccal mass protractor muscle (I2) in Aplysia. J Neurophysiol 84(6):2810–2820
Kerkut GA, Lambert JDC, Gayton RJ, Loker JE, Walker RJ (1975) Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Phys 50A:1–25
Kier WM (1992) Hydrostatic skeletons and muscular hydrostats (Biomechanics (structures and systems): a practical approach). Oxford University Press, New York
Kobayashi M, Muneoka Y, Fujiwara M (1981) The modulatory actions of the possible neurotransmitters in the molluscan radular muscles. In: Rózsa KS (ed) Advanced physiological science, vol 22. Pergamon Press, Oxford, pp 319–337
Krajcs N, Márk L, Elekes K, Kiss T (2012) Morphology, ultrastructure and contractile properties of muscles responsible for superior tentacle movements of the snail. Acta Biol Hung 63(Suppl 2):129–140
Lemaire M, Chase R (1998) Twitching and quivering of the tentacles during snail olfactory orientation. J Comp Physiol 182:81–87
Magoski NS, Bulloch AG (1999) Dopamine activates two different receptors to produce variability in sign at an identified synapse. J Neurophysiol 81(3):1330–1340
Moore PA, Atema J, Gerhard GA (1991) Fluid dynamics and microscale chemical movement in the chemosensory appendages of the lobster, Homarus americanus. Chem Sens 16:663–674
Nikitin ES, Zakharov IS, Samarova EI, Kemenes G, Balaban PM (2005) Fine tuning of olfactory orientation behaviour by the interaction of oscillatory and single neuronal activity. Eur J Neurosci 22:2833–2844
Peschel M, Straub V, Teyke T (1996) Consequences of food-attraction conditioning in Helix: a behavioral and electrophysiological study. J Comp Physiol A 178:317–327
Rogers DC (1968) Fine structure of smooth muscle and neuromuscular junctions in the optic tentacles of Helix aspersa and Limax flavus. Z Zellforsch 89:80–94
Rogers DC (1969) Fine structure of smooth muscle and neuromuscular junctions in the foot of Helix aspersa. Z Zellforsch 99:315–335
Sherman RG, Fourtner CR, Drewes CD (1976) Invertebrate nerve-muscle systems. Comp Biochem Physiol 53A:227–233
Weiss KR, Cohen JL, Kupfermann I (1978) Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol 41(1):181–203
Yoshida M, Kobayashi M (1991) Neural control of the buccal muscle movement in the African giant snail Achatina fulica. J Exp Biol 155:415–433
Zakharov IS (1992) Avoidance behavior of the snail. J High Nerv Act 42:1156–1169 (in Russian)
Zoran MJ, Haydon PG, Matthews PJ (1989) Aminergic and peptidergic modulation of motor function at an identified neuromuscular junction in Helisoma. J Exp Biol 142:225–243
Acknowledgments
Authors express their gratitude to H. Kimura providing the cChAT antisera and Jean-Pierre Bellier (Molecular Neuroscience Research Center, Shiga University of Medical Science, Seta Tsukinowa-cho, Otsu, Shiga 520-2192 Japan) for the mediation our request to H. Kimura. This work was supported by grants from the Hungarian Scientific Research Fund (OTKA) No. 782248 (KE).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that the experiments comply with the current ethical laws of Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krajcs, N., Hernádi, L., Elekes, K. et al. Excitatory neurotransmitters in the tentacle flexor muscles responsible for space positioning of the snail olfactory organ. Invert Neurosci 14, 59–69 (2014). https://doi.org/10.1007/s10158-013-0164-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-013-0164-y