Abstract
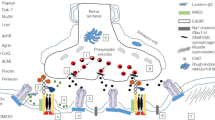
Mutations affecting acetylcholine receptors have been causally linked to the development of congenital myasthenic syndromes (CMS) in humans resulting from neuromuscular transmission defects. In an undergraduate Molecular Neurobiology course, the molecular basis of CMS was explored through study of a Caenorhabditis elegans model of the disease. The nicotinic acetylcholine receptor (nAChR), located on the postsynaptic muscle cell membrane, contains a pentameric ring structure comprised of five homologous subunits. In the nematode C. elegans, unc-63 encodes an α subunit of nAChR. UNC-63 is required for the function of nAChR at the neuromuscular junction. Mutations in unc-63 result in defects in locomotion and egg-laying and may be used as models for CMS. Here, we describe the responses of four unc-63 mutants to the cholinesterase inhibitor pyridostigmine bromide (range 0.9–15.6 mM in this study), a treatment for CMS that mitigates deficiencies in cholinergic transmission by elevating synaptic ACh levels. Our results show that 15.6 mM pyridostigmine bromide enhanced mobility in two alleles, depressed mobility in one allele and in N2, while having no effect on the fourth allele. This indicates that while pyridostigmine bromide may be effective at ameliorating symptoms of CMS in certain cases, it may not be a suitable treatment for all individuals due to the diverse etiology of this disease. Students in the Molecular Neurobiology course enhanced their experience in scientific research by conducting an experiment designed to increase understanding of genetic defects of neurological function.


Similar content being viewed by others
References
Baraka A, Mansour A, Haddad W, Wakid N (1981) Effect of neostigmine and pyridostigmine on the plasma cholinesterase activity. Br J Anaesth 53:849–851
Bieri T, Blasiar D, Ozersky P, Antoshechkin I, Bastiani C, Canaran P, Chan J, Chen N, Chen WJ, Davis P, Fiedler TJ, Girard L, Han M, Harris TW, Kishore R, Lee R, McKay S, Müller HM, Nakamura C, Petcherski A, Rangarajan A, Rogers A, Schindelman G, Schwarz EM, Spooner W, Tuli MA, Auken KV, Wang D, Wang X, Williams G, Durbin R, Stein LD, Sternberg PW, Spieth J (2007) Wormbase: new content and better access. Nucleic Acids Res 35:D506–D510
Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau J-L (2008) Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci 105(47):18590–18595
Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van der Oost J, Smit AB, Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand binding domain of nicotinic receptors. Nature 411:269–276
Brenner S (1974) The Genetics of Caenorhabditis elegans. Genetics 77:71–94
Breyer-Pfaff U, Maier U, Brinkmann AM, Schumm F (1985) Pyridostigmine kinetics in healthy subjects and patients with myasthenia gravis. Clin Pharmacol Ther 37(5):495–501
Breyer-Pfaff U, Schmezer A, Maier U, Brinkmann A, Schumm F (1990) Neuromuscular function and plasma drug levels in pyridostigmine treatment of myasthenia gravis. J Neurol Neurosurg Psychiatry 53:502–506
Brown LA, Jones AK, Buckingham SD, Mee CJ, Sattelle DB (2006) Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study. Int J Parasitol 36:617–624
Cooper E, Couturier S, Ballivet M (1991) Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature 350:235–238
Culletto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, Lewis JA, Sattelle DB (2004) The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor α subunit. J. Biol. Chem. 279:42476–42483
Dunckley T, Wu J, Zhao L, Lukas RJ (2003) Mutational analysis of roles for extracellular cysteine residues in the assembly and function of human α7-nicotinic acetylcholine receptors. Biochemistry 42:870–876
Engel AG, Uchitel OD, Walls TJ, Nagel A, Harper CM, Bodensteiner J (1993) Newly recognized congenital myasthenic syndrome associated with high conductance and fast closure of the acetylcholine receptor channel. Ann Neurol 34:38–47
Engel AG, Ohno K, Milone M, Wang H-L, Nakano S, Bouzat C, Pruitt JN, Hutchinson DO, Brengman JM, Bren N, Sieb JP, Sine SM (1996) New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum Mol Genet 5:1217–1227
Green WN, Wanamaker CP (1997) The role o f the cystine loop in acetylcholine receptor assembly. J Biol Chem 272:20945–20953
Hutchinson DO, Walls TJ, Nakano S, Camp S, Taylor P, Harper CM, Groover RV, Peterson HA, Jamieson DG, Engel AG (1993) Congenital endplate acetylcholinesterase deficiency. Brain 116:633–653
Jones AK, Sattelle DB (2004) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. BioEssays 26:39–49
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Bio 305:567–580
Lewis JA, Wu CH, Berg H, Levine JH (1980) The genetics of levamisole resistance in Caenorhabditis elegans. Genetics 95:905–928
Mirakhur RK, Lavery TD, Briggs LP, Clarke RSJ (1982) Effects of neostigmine and pyridostigmine on serum cholinesterase activity. Can Anaesth Soc J 29:55–58
Mora M, Lambert EH, Engel AG (1987) Synaptic vesicle abnormality in familial infantile myasthenia. Neurology 37:206–214
Nguyen M, Alfonso A, Johnson CD, Rand JB (1995) Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140(2):527–535
Pascuzzo GJ, Akaike A, Maleque MA, Shaw KP, Aronstam RS, Rickett DL, Albuquerque EX (1984) The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex I. Agonist, desensitizing, and binding properties. Mol Pharmacol 25:92–101
Rand JB, Russell RL (1984) Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 106(2):227–248
Sattelle DB, Culetto E, Baylis H (2000) International Publication No.WO 00/75311 Al. World Intellectual Property Organization: International Bureau
Schara U, Lochmuller H (2008) Therapeutic strategies in congenital myasthenic syndromes. Neurotheratpeutics 5:542–547
Shen XM, Deymeer F, Sine SM, Engel AG (2006) Slow-channel mutation in acetylcholine receptor αM4 domain and its efficient knockdown. Ann Neurol 60:128–136
Sine SM, Ohno K, Bouzat C, Auerbach A, Milone M, Pruitt JN, Engel AG (1995) Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron 15(1):229–239
Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, Richmond JE (2005) acr-16 encodes an essential subunit of the levamisole-resistant receptor at the Caenorhabditis elegans neuromuscular junction. JBC 280:27013–27021
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4 A° resolution. J Mol Biol 346:967–989
Unwin N, Miyazawa A, Li J, Fujiyoshi Y (2002) Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J Mol Biol 319:1165–1176
Walls TJ, Engel AG, Nagel AS, Harper CM, Trasek VF (1993) Congenital myasthenic syndrome associated with paucity of synaptic vesicles and reduced quantal release. Ann NY Acad Sci 37:206–214
Wang Z-Z, Hardy SF, Hall ZW (1996) The first transmembrane domains of truncated α and δ subunits are required for heterodimer formation in vivo. JBC 271:27575–27584
Webster R, Brydson M, Croxen R, Newsom-Davis J, Vincent A, Beeson D (2004) Mutation in the AChR ion channel gate underlies a fast channel congenital myasthenic syndrome. Neurology 62:1090–1096
Acknowledgments
We thank David Sattelle for the inspiration for these laboratory experiments. Students in the Molecular Neurobiology course at Ursinus College all participated in planning and executing experiments and in interpreting experimental results. These students included, Brigid Bleaken, Lauren Brady, Ashley Feigenbutz, Laura Gurenlian, Danielle Indelicato, Krithika Krishnarao, Cierra Lewis, Elizabeth Marion, Lauren Myers, Bindu Pirlamarla, Stephanie Rogman, Noelle Romero, Larissa Stuckey, Nicholas Turner, Douglas Yodice, and Jonathan Zhou. We thank Kenton Woodard for making stock solutions and organizing laboratory materials. We thank Roger Coleman for his assistance with statistical analysis. C. elegans strains were provided by the C. elegans Genetics Center. This material is based upon work supported by the National Science Foundation under Grant No. 0544031 to REK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaas, B., Vaidya, A.R., Leatherman, A. et al. Technical report: exploring the basis of congenital myasthenic syndromes in an undergraduate course, using the model organism, Caenorhabditis elegans . Invert Neurosci 10, 17–23 (2010). https://doi.org/10.1007/s10158-010-0101-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-010-0101-2