Abstract
Background
The present study aimed to obtain information enabling optimisation of the clinical effect of mizoribine (MZR) in pediatric patients with kidney disease.
Methods
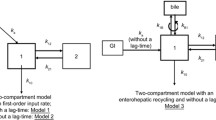
A total of 105 pediatric patients with kidney disease treated at our institutions were enrolled. Kidney transplant patients were excluded. Population pharmacokinetic analysis of MZR was performed based on serum concentration data. Area under the curve from time zero to infinity (AUC∞) and maximal concentration (C max) were calculated by Bayesian analysis.
Results
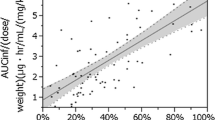
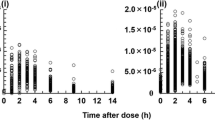
In children, the appearance of MZR in the blood tended to be slower and the subsequent rise in blood concentration tended to be more sluggish, compared to healthy adults. Apparent volume of distribution and oral clearance were also higher in children compared to adults. A significant positive correlation was observed between patient age and AUC∞. There were significant differences of AUC∞ and C max by age group. No relationship was observed between the administration method of MZR and serum concentration.
Conclusion
The pharmacokinetics of MZR was different in children compared to adults. To obtain the expected clinical efficacy, the regular MZR dosage schedule (2–3 mg/kg/day) might be insufficient for pediatric patients. In particular, younger patients might require a higher dosage of MZR per unit body weight.



Similar content being viewed by others
References
Yokota S. Mizoribine: mode of action and effects in clinical use. Pediatr Int. 2002;44:196–8.
Kawasaki Y. Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol. 2009;2009:681482.
Sonda K, Takahashi K, Tanabe K, Funchinoue S, Hayasaka Y, Kawaguchi H, et al. Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc. 1996;28:3643–8.
Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int. 2000;58:317–24.
Honda M. Nephrotic syndrome and mizoribine in children. Pediatr Int. 2002;44:210–6.
Kawasaki Y, Hosoya M, Suzuki J, Onishi N, Takahashi A, Isome M, et al. Efficacy of multidrug therapy combined with mizoribine in children with diffuse IgA nephropathy in comparison with multidrug therapy without mizoribine and with methylprednisolone pulse therapy. Am J Nephrol. 2004;24:576–81.
Yoshikawa N, Nakanishi K, Ishikura K, Hataya H, Iijima K, Honda M. Combination therapy with mizoribine for severe childhood IgA nephropathy: a pilot study. Pediatr Nephrol. 2008;23:757–63.
Tanaka H, Tsugawa K, Tsuruga K, Suzuki K, Nakahata T, Ito E, et al. Mizoribine for the treatment of lupus nephritis in children and adolescents. Clin Nephrol. 2004;62:412–7.
Yumura W, Suganuma S, Uchida K, Moriyama T, Otsubo S, Takei T, et al. Effects of long-term treatment with mizoribine in patients with proliferative lupus nephritis. Clin Nephrol. 2005;64:28–34.
Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Ito E, Waga S. Mizoribine oral pulse therapy for patients with disease flare of lupus nephritis. Clin Nephrol. 2003;60:390–4.
Tanaka H, Tsugawa K, Suzuki K, Nakahata T, Ito E. Long-term mizoribine intermittent pulse therapy for young patients with flare of lupus nephritis. Pediatr Nephrol. 2006;21:962–6.
Kuroda T, Hirose S, Tanabe N, Sato H, Nakatsue T, Ajiro J, et al. Mizoribine therapy for patients with lupus nephritis: the association between peak mizoribine concentration and clinical efficacy. Mod Rheumatol. 2007;17:206–12.
Tanaka H, Oki E, Tsuruga K, Sato N, Matsukura H, Matsunaga A, et al. Mizoribine treatment of young patients with severe lupus nephritis: a clinicopathologic study by the Tohoku pediatric study group. Nephron Clin. Pract. 2008;110:c73–9.
Ohtomo Y, Fujinaga S, Takada M, Murakami H, Akashi S, Shimizu T, et al. High-dose mizoribine therapy for childhood onset frequently relapsing steroid-dependent nephrotic syndrome with cyclosporin nephrotoxicity. Pediatr Nephrol. 2005;20:1744–9.
Kawasaki Y, Takano K, Isome M, Suzuki J, Suyama K, Kanno H, et al. Efficacy of single dose of oral mizoribine pulse therapy two times per week for frequently relapsing nephrotic syndrome. J Nephrol. 2007;20:52–6.
Mahmood I, Miller R. Comparison of Bayesian approach and a limited sampling model for the estimation of AUC and Cmax: a computer simulation analysis. Int J Clin Pharmacol Ther. 1999;37:439–45.
Honda M, Itoh H, Suzuki T, Hashimoto Y. Population pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Biol Pharm Bull. 2006;29:2460–4.
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37.
Hosotsubo H, Takahara S, Tanaka N. Simplified high-performance liquid chromatographic method for determination of mizoribine in human serum. J Chromatogr. 1988;432:340–5.
Beal S, Boeckmann A, Sheiner L. NONMEM users guides: NONMEM Project Group. San Francisco: University of California; 1992.
Stypinski D, Obaidi M, Combs M, Weber M, Stewart AJ, Ishikawa H. Safety, tolerability and pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Br J Clin Pharmacol. 2007;63:459–68.
Okada M, Suzuki K, Nakashima M, Nakanishi T, Fujioka N. The nucleotide derivatives inosine and inosinic acid inhibit intestinal absorption of mizoribine in rats. Eur J Pharmacol. 2006;531:140–4.
Ishida K, Takaai M, Yotsutani A, Taguchi M, Hashimoto Y. Membrane transport mechanisms of mizoribine in the rat intestine and human epithelial LS180 cells. Biol Pharm Bull. 2009;32:741–5.
Acknowledgments
We thank Asahi Kasei Pharma, Tokyo, for measuring serum MZR concentrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
About this article
Cite this article
Kaneda, H., Shimizu, M., Ohta, K. et al. Population pharmacokinetics of mizoribine in pediatric patients with kidney disease. Clin Exp Nephrol 20, 757–763 (2016). https://doi.org/10.1007/s10157-015-1209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1209-9