Abstract
Background
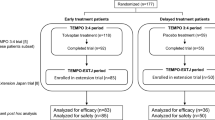
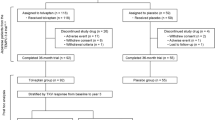
Japan is the first country in the world to approve tolvaptan for the treatment of autosomal dominant polycystic kidney disease (ADPKD), which was based on the results of Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 trial. To evaluate the safety and efficacy of tolvaptan, we performed a subgroup analysis in the participating Japanese ADPKD patient population.
Methods
The primary outcome was the annual rate of percentage change in the total kidney volume (TKV). The secondary endpoint was the rate of kidney function change.
Results
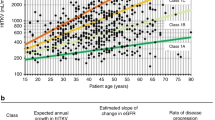
The tolvaptan and placebo groups included 118 and 59 patients, respectively. The annual rate of percentage changes in TKV were 1.3 % [95 % confidence interval (CI) 0.4–2.1] in the tolvaptan group, and 5.0 % (95 % CI 3.9–6.2) in the placebo group (P < 0.001). The annual estimated glomerular filtration rate change was −3.83 mL/min/1.73 m2 in the tolvaptan group and −5.05 mL in the placebo group for a treatment effect of +1.22 mL/min/1.73 m2 (95 % CI 0.41–2.02: P = 0.003). Hepatic function abnormal as a serious adverse event was observed in 3 patients (2.5 %) in the tolvaptan group.
Conclusions
Administration of tolvaptan in the Japanese sub-population reduced the annual rate of TKV growth and slowed the rate of kidney function decline over 36 months compared to patients on placebo, thus providing a novel and effective therapy for the treatment of ADPKD. (TEMPO 3:4 ClinicalTrials.gov number, NCT00428948).









Similar content being viewed by others
References
Higashihara E, Nutahara K, Kojima N, et al. Prevalence and renal prognosis of diagnosed Autosomal-dominant polycystic kidney disease in Japan. Nephron. 1998;80:421–7.
Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 2000;58:925–43.
Parikh CR, Dahl NK, Chapman AB, et al. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int. 2012;81:784–90.
Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30.
Hughes J, Ward CJ, Peral B, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–60.
Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–42.
Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 study. Am J Kidney Dis. 2011;57:692–9.
Masoumi A, Reed-Gitomer B, Kelleher C, et al. Developments in the management of autosomal dominant polycystic kidney disease. Ther Clin Risk Manag. 2008;4:393–407.
Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol. 2013;17:317–26.
Wang X, Gattone V II, Harris PC, et al. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–51.
Gattone VH II, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–6.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–18.
The International Society of Nephrology. Summary of recommendation statements. Kidney Int Suppl. 2013;3:5–14.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Serra A, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–9.
Matsuzaki M, Hori M, Izumi T, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther. 2011;25(Suppl 1):S33–45.
Sakaida I, Kawazoe S, Kajimura K, et al. Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82.
Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68.
Acknowledgments
Data analyzed in the present subgroup analysis were all provided by Otsuka Pharmaceutical Development and Commercialization (Maryland, USA) to Department of Medical Affairs (Otsuka Pharmaceutical Co., Ltd. Tokyo, Japan). The authors’ sincere thanks are due to Taku Seriu and Kyoji Imaoka for approval of this project and generous support, are due to Osamu Sato, Tadashi Okada, Mitsuhiro Kondo, Saki Misono, Emi Tomotake and Yoshitaka Yamamura for implementation of the TEMPO 3:4 trial in Japan, and are due to Jaime Blais, Koji Nakajima, Yoshiyuki Shibasaki, Hidetsugu Tsubouchi, Kazumasa Kishi, Hiromi Oka, Sayaka Tachikawa, and Hiroyuki Kobayashi for interpretation of the data, and review and revision of the present manuscript. These study management and editorial assistance were all provided by Department of Clinical Development (Otsuka) and Department of Medical Affairs.
Conflict of interest
SH has received honoraria for presentations and research funding from and has consulted and been an advisory board member for Otsuka Pharmaceutical. EH has received research funding from Otsuka Pharmaceutical and is a member of the Steering Committee for several Otsuka studies involving the TEMPO 3:4 trial. TM and JO are employees of Otsuka. VET is an investigator and Chair of the Steering Committee for several Otsuka studies involving the TEMPO 3:4 trial. All the other authors declared no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Muto, S., Kawano, H., Higashihara, E. et al. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin Exp Nephrol 19, 867–877 (2015). https://doi.org/10.1007/s10157-015-1086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1086-2