Abstract
Background
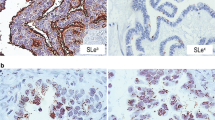
Changes in expression patterns of the sialyl Lewis antigens and MUC1 mucin can be considered as markers for the diagnosis of various cancers. However, there are no reports which have been devoted to analysis of differences in the sialyl Lewis antigens and MUC1 expression patterns as potential discrimination markers among different areas of clear cell renal cell carcinoma (ccRCC). The aim of this study was to determine the level of MUC1 and specific Lewis antigens on glycoproteins in three different areas: tumor (cancer tissue), intermediate zone (adjacent to tumor tissue) and normal renal cortex/medulla (uninvolved by tumor).
Methods
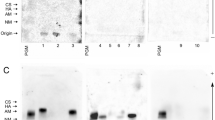
Study was performed on renal tissues taken from 30 patients with clear cell renal cell carcinoma. Relative amounts of sugar structures bound with proteins were determined by ELISA-like test with biotinylated lectins or monoclonal antibodies: anti-MUC1 and anti-sialyl Lewisa/x. The study presented here provides novel information about relationship between MUC1 and sialyl Lewis antigens in the tumor, intermediate zone and noninvolved areas of normal renal tissue distant of tumor.
Results
We have found statistically significant increase of MUC1 and sialic acid linked by α-2,3 bond with galactose in cancer tissue and in intermediate zone comparing to normal renal tissue distant of tumor. Moreover, we observed statistically significant increase of sialic acid linked by α-2,6 bond with Gal/GalNAc and sialyl Lewisa/x antigens in cancer tissues only, comparing to normal ones.
Conclusions
MUC1 and sialylated antigens can be involved in renal tumor development and can be considered as potential markers distinguishing normal renal tissue from intermediate zone and from cancer renal cells during ccRCC development.


Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- ccRCC:
-
Clear cell renal cell carcinoma
- ELISA:
-
Enzyme-linked immunosorbent assay
- Gal:
-
Galactose
- GalNAc:
-
N-acetylgalactosamine
- GlcNAc:
-
N-acetylglucosamine
- MAA:
-
Maackia Amurensis Agglutinin
- sLea :
-
Sialyl Lewisa antigen
- sLex :
-
Sialyl Lewisx antigen
- SNA:
-
Sambucus Nigra Agglutinin
References
Saeland E, Belo AI, Mongera S, van Die I, Meijer GA, van Kooyk Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int J Cancer. 2012;131:117–28.
Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;98:74–85.
Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–81.
Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61–9.
Aubert S, Fauquette V, Hémon B, Lepoivre R, Briez N, Bernard D, Van Seuningen I, Leroy X, Perrais M. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res. 2009;69:5707–15.
Swallow DM, Gendler S, Griffiths B, Kearney A, Povey S, Sheer D, Palmer RW, Taylor-Papadimitriou J. The hypervariable gene locus PUM, which codes for the tumour associated epithelial mucins is located on chromosome 1, within the region 1q21-24. Ann Hum Genet. 1987;51:289–94.
Bouillez A, Gnemmi V, Gaudelot K, Hémon B, Ringot B, Pottier N, Glowacki F, Butruille C, Cauffiez C, Hamdane M, Sergeant N, Van Seuningen I, Leroy X, Aubert S, Perrais M. MUC1-C nuclear localization drives invasiveness of renal cancer cells through a sheddase/gamma secretase dependent pathway. Oncotarget. 2014;5:754–63.
Leroy X, Zerimech F, Zini L, Copin MC, Buisine MP, Gosselin B, Aubert JP, Porchet N. MUC1 expression is correlated with nuclear grade and tumor progression in pT1 renal clear cell carcinoma. Am J Clin Pathol. 2002;118:47–51.
Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–34.
Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasias. 2002;19:339–45.
Singh PK, Hollingsworth MA. Cell-surface associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–76.
Wang PH. Altered Sialylation and Its Roles in Gynecologic Cancers. J Cancer Mol. 2006;2:107–16.
Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–99.
Sawada T, Ho JJ, Sagabe T, Yoon WH, Chung YS, Sowa M, Kim YS. Biphasic effect of cell surface sialic acids on pancreatic cancer cell adhesiveness. Biochem Biophys Res Commun. 1993;195:1096–103.
Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–3.
Zhao J, Patwa TH, Qiu W, Shedden K, Hinderer R, Misek DE, Anderson MA, Simeone DM, Lubman DM. Glycoprotein microarrays with multi-lectin detection: unique lectin binding patterns as a tool for classifying normal, chronic pancreatitis and pancreatic cancer sera. J Proteome Res. 2007;6:1864–74.
Dall’Olio F, Malagolini N, di Stefano G, Minni F, Marrano D, Serafini-Cessi F. Increased CMP-NeuAc:Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity in human colorectal cancer tissues. Int J Cancer. 1989;44:434–9.
Kaneko Y, Yamamoto H, Kersey DS, Colley KJ, Leestma JE, Moskal JR. The expression of Gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2,6-linked sialoglycoconjugates in human brain tumors. Acta Neuropathol. 1996;91:284–92.
Tian Y, Esteva FJ, Song J, Zhang H. Altered expression of sialylated glycoproteins in breast cancer using hydrazide chemistry and mass spectrometry. Mol Cell Proteomics. 2012;11(M111):011403.
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85.
Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76.
Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–904.
Ghosh SK, Pantazopoulos P, Medarova Z, Moore A. Expression of underglycosylated MUC1 antigen in cancerous and adjacent normal breast tissues. Clin Breast Cancer. 2013;13:109–18.
Von Mensdorff-Pouilly S, Snijdewint FG, Verstraeten AA, Verheijen RH, Kenemans P. Human MUC1 mucin: a multifaceted glycoprotein. Int J Biol Markers. 2000;15:343–56.
Cao Y, Karsten U, Zerban H, Bannasch P. Expression of MUC1, Thomsen-Friedenreich-related antigens, and cytokeratin 19 in human renal cell carcinomas and tubular clear cell lesions. Virchows Arch. 2000;436:119–26.
Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–17.
Cordon-Cardo C, Reuter VE, Finstad CL, Sheinfeld J, Lloyd KO, Fair WR, Melamed MR. Blood group-related antigens in human kidney: modulation of Lewis determinants in renal cell carcinoma. Cancer Res. 1989;49:212–8.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Borzym-Kluczyk, M., Radziejewska, I. & Cechowska-Pasko, M. Increased expression of MUC1 and sialyl Lewis antigens in different areas of clear renal cell carcinoma. Clin Exp Nephrol 19, 732–737 (2015). https://doi.org/10.1007/s10157-014-1013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-014-1013-y