Abstract
Background
Hyperglycemia/high glucose may induce apoptosis in diabetic kidney, but the mechanism is not fully understood. Naofen was found as a Shiga toxin (Stx)-2-related protein. Based on renal dysfunction in infection with Stx-producing Escherichia coli and on participation of naofen in apoptosis of human embryonic kidney cells, the present study was undertaken to investigate the mechanism of renal dysfunction in diabetes mellitus with particular reference to naofen.
Methods
In in vivo studies utilizing streptozotocin (STZ)-induced diabetic rats, and also in in vitro cultured rat kidney epithelial (NRK52E) cells, naofen messenger RNA (mRNA) and protein expressions were analyzed. Naofen mRNA location in diabetic kidney was studied by in situ hybridization. Apoptosis was assessed by caspase-3 activity assay.
Results
Rat diabetic kidney showed significant increases in caspase-3 activities and naofen mRNA. Naofen was mainly observed at both proximal and distal urinary tubules. Incubation of NRK52E cells in high glucose medium resulted in elevated naofen mRNA expression, whereas neither interleukin-1, interleukin-6, nor tumor necrosis factor-α elicited such action. Moreover, treatment of NRK52E cells with naofen small interfering RNA (siRNA) inhibited naofen mRNA expression induced by high glucose and blocked the increase in caspase-3 activity.
Conclusions
These data suggest that naofen expression may be upregulated by hyperglycemia, with possible correlation to apoptosis of tubular epithelial cells and thereby to diabetic nephropathy.
Similar content being viewed by others
Introduction
Diabetic pandemic is a major cause of morbidity and mortality worldwide [1, 2], diabetic nephropathy being the largest single cause of end-stage renal disease [3, 4]. An emerging area of research has focused on the role of apoptosis in mediating some of the effects of diabetes [5]. High concentration of glucose (high glucose)-induced apoptosis has received much attention in recent years, but the mechanism remains unclear. Among them, in the mitochondrial intramembrane space, cytochrome c may mediate the allosteric activation and hepta-oligomerization of the adaptor molecule apoptosis-protease activating factor 1 (Apaf-1), a WD-repeat (tryptophan-aspartate repeat) protein, in the presence of adenosine triphosphate (ATP), and generate a complex known as the apoptosome [6]. Each apoptosome can recruit seven dimers of caspase-9, promoting the activation of the enzymes, leading to proteolytic self-processing and subsequently catalytic maturation of caspase-3 and biochemical and morphological features of apoptosis [7].
Naofen (GenBank access no. EF613262) is a novel WD-repeat protein of 1170 amino acids found by cloning the brain/spinal cord complementary DNA (cDNA) library of rats, as a reactive substance to an antibody against the Shiga toxin (Stx)-2 released from Stx-producing Escherichia coli (STEC) [8]. Since Stx-2 may induce apoptosis of many types of cells, including renal tubular epithelial cells as well as glomerular capillary endothelial cells [9, 10], and vascular endothelial cells [11], we hypothesized that naofen may also be related to apoptosis. Thus, recently we have reported that naofen may possibly participate in apoptosis via activating caspase-3, and additionally that naofen siRNA inhibited apoptosis induced by tumor necrosis factor (TNF)-α in human embryonic kidney (HEK293) cells (unpublished observation), suggesting that naofen induced by TNF-α may mediate activation of caspases.
The present study was undertaken to evaluate the roles of naofen especially in the kidney of streptozotocin (STZ)-induced diabetic model of rats and also in rat kidney epithelial (NRK52E) cell line cultured in high glucose medium.
Materials and methods
Animal experimental protocol and tissue preparation
All animal experiments were conducted in accordance with our institutional guidelines for animal research. Male Wistar rats (aged 8 weeks), weighing 200–220 g at the start of the experiment, were purchased from SLC (Shizuoka, Japan) and allowed free access to food and water.
Diabetes was induced by intraperitoneal injection of 65 mg/kg streptozotocin (STZ; Sigma–Aldrich, USA) prepared in 0.1 M citrate buffer (pH 4.5), after overnight fast. Control rats were injected with only an equal volume of citrate buffer. Diabetes was confirmed at 48 h after STZ injection; rats with blood glucose levels more than 300 mg/dl were selected and used for this study. Rats were killed at 8, 12, 16, and 24 weeks after STZ injection. After anesthesia with pentobarbital sodium (50 mg/kg, i.p.), right-side kidney samples were stored in liquid nitrogen for mRNA analyses. Blood was washed out with saline, and then 4% paraformaldehyde (PFA) solution dissolved with phosphate-buffered saline (PBS) was infused into the circulation via the left ventricle. Then, left-side kidney tissues were transversally sliced and fixed in 4% PFA solution for 24 h, being then embedded in paraffin, cut to 5 μm thickness, and used for histological analyses.
In situ hybridization (ISH) assay
In situ hybridization was performed in an incubator (Micro Probe Incubator; Fisher Biotech, Fisher Scientific, Pittsburgh, PA, USA), using the procedure provided by the manufacturer, with a probe produced as biotin-labeled Brigati-tailed probe (Falma, Tokyo, Japan). The sequence of the naofen probe was as follows: 5′-GCAGAAAGGATATATGCTCTGTGTACTTTA-3′. Hybridization was performed at 105°C for 5 min and 74°C for 60 min, with a probe diluted at 1:2500. Positive regions were visualized with diaminobenzidine (DAB; Falma, Tokyo, Japan), thus resulting in brown staining, and then counterstained with hematoxylin for microscopic examination.
Western blot analysis
Samples from kidney were homogenized in lysis buffer [50 mM Tris–HCl, pH 7.4, 1 mM ethylenediamine tetraacetic acid (EDTA), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100, 10% glycerol] containing a mixture of protease inhibitors (CompleteTM tables; Roche Applied Sciences, Mannheim, Germany) and incubated for 1 h on ice. Cell lysates were centrifuged for 10 min at 4°C at 15,000 × g, and protein concentrations of samples were determined by utilizing DC protein assay (Bio-Rad Laboratories, Hercules, CA). Protein levels of naofen and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were analyzed by Western blotting with anti-naofen638-656 peptide antibody, anti-naofen1104-1120 peptide antibody (1:100; produced and purified by Peptide Institute, Osaka, Japan), and anti-GAPDH antibody (1:500; Santa Cruz, CA, USA). Detection was performed with Western blotting reagent ECL (GE healthcare, Buckinghamshire, UK). The protein level was quantified by densitometric scanning with Gel-Pro Analyzer (Media Cybernetics, Inc., USA) and expressed as the ratio to GAPDH as described previously [12].
Apoptosis analysis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptosis was evaluated by utilizing the TUNEL assay. TUNEL staining using the ApopTag® Plus peroxidase in situ apoptosis detection kit (Millipore Corporation, Billerica, MA, USA) was performed according to the manufacturer’s instructions. The number of TUNEL-positive cells was counted in five randomly selected fields of view under 100× magnification for each animal, and six animals were studied per group.
Cell line culture experiments
Normal rat kidney epithelial (NRK52E) cells were purchased from the Health Science Research Resources Bank (Tokyo, Japan). NRK52E cells were maintained in normal-concentration glucose (5 mM) Dulbecco’s Minimal Essential Medium (DMEM; Wako, Osaka, Japan) containing 10% heat-inactivated fetal bovine serum with 100 units penicillin and 100 μg/ml streptomycin (Gibco BRL, Grand Island, NY, USA). Cells were maintained at 37°C in humid air with 5% CO2. The culture medium was replaced every 2–3 days. To prepare the cell suspension, cells were treated with trypsin (0.25%)-EDTA (1 mM) (Gibco BRL, Grand Island, NY, USA), then transferred into a 6-cm culture plate at 1 × 105 cells, and cultured overnight.
To determine the relevance of naofen in diabetic nephropathy, naofen mRNA expressions were measured in NRK52E cells maintained in DMEM containing either 5 or 25 mM glucose, in the absence or presence of TNF-α (30 ng/ml; PeproTech Inc., Rocky Hill, USA) for 18 h. In order to examine whether the effects of high glucose on naofen expression were induced by high osmotic pressure, naofen expression was analyzed under high osmotic pressure with 25 mM mannitol (Wako, Osaka, Japan). Experiments were performed in triplicate and repeated seven times.
siRNA transfection and naofen knockdown
Small interfering RNA (siRNA) for rat naofen (NF-siRNA, siRNA ID: s235456) and control-siRNA (negative control#1 siRNA, catalog no. 4390843) were purchased from Ambion (Austin, TX, USA). The sequence of NF-siRNA was as follows: 5′-GGAUGAAUGCUGUACAAUAtt-3′ (sense), 5′-UAUUGUACAGCAUUCAUCCac-3′ (antisense). The negative control siRNA is a circular plasmid encoding a hairpin siRNA, whose sequence is not found in the mouse, human, or rat genome databases.
Transfection of NF-siRNA (final concentration 10 nM) or control-siRNA (final concentration 10 nM) was performed by using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. At 24 h after transfection with NF-siRNA, the knockdown efficiencies of NF-siRNA were verified at the RNA level by real-time polymerase chain reaction (PCR). In experiments with high glucose treatment, high glucose medium was added at 24 h post transfection and cultured for another 18 h until caspase-3 activity assays were performed. At least six experiments were performed independently for each siRNA assay.
Quantitative real-time PCR (qPCR) analysis
Total RNA (1 μg), extracted using TRIzol® reagent (Invitrogen, USA) from kidney tissues of rat or cultured NRK52E cells, was reversely transcribed using a ReverTra Ace® qPCR RT kit (Toyobo, Osaka, Japan). qPCR was performed with an ABI StepOne Plus real-time PCR system using the TaqMan detection gene expression assay (Applied Biosystems, Tokyo, Japan) according to the manufacturer’s instruction. The primers and TaqMan MGB probe sets for rat naofen, which was designed by Applied Biosystems (Custom TaqMan® Genomic Assay Service), were 5′-CGATTTCTGCATTTTGGCCACAA-3′ (forward primer), 5′-TCCAAGGGTGTGCCAATAGAATT-3′ (reverse primer), and 5′-CAAACTGAGGGTGATTTT-3′ (TaqMan MGB probe). The relative abundance of the target was obtained by calculating against a standard curve and normalized to internal control (GAPDH, Rn99999916_sl) compared with the control group according to the ΔΔC T method as previously reported [13].
Measurement of caspase-3 activities
Activities of caspase-3 in the kidney of diabetic rats or cultured NRK52E cells were determined using the caspase-3/CPP32 fluorometric assay kit (Medical & Biological Laboratories, Nagoya, Japan) according to the manufacturer’s instruction. These assays are based on detection of the cleavages of a specific fluorometric caspase substrate, which are DEVD (Asp-Glu-Val-Asp)-AFC (7-amino-4-trifluoromethyl coumarin) for caspase-3. In brief, samples were homogenized in cell lysis buffer supplied in the kit. Samples (200 μg protein) were mixed with 2× reaction buffer [containing 10 mM dithiothreitol (DTT)]. After incubation at 37°C for 2 h, free AFC cleaved by caspase from specific substrates was quantified by Fluoroskan Ascent FL (Labsystems, Helsinki, Finland) with excitation/emission (Ex/Em) = 400/505 nm as previously reported [14].
Statistical analysis
All results are expressed as mean ± standard error of the mean (SEM), and were analyzed for statistical significance by Kruskal–Wallis one-way analysis of variance (ANOVA). Differences were considered significant at P < 0.05.
Results
Establishment of the diabetic nephropathy model
After one-shot injection of streptozotocin (STZ), body weight and blood glucose concentrations were recorded until the time of killing animals. Blood glucose levels in the diabetic rats were significantly increased after STZ injection, leveling off at the fourth week (Fig. 1a). Body weights in the acutely induced diabetic group were significantly lower than those obtained in the control group (Fig. 1b). Hematoxylin and eosin (H&E) staining showed glomerular hypertrophy, thickening of the glomerular basement membrane, tubular atrophy, and interstitial fibrosis in diabetic kidney (data not shown).
Naofen mRNA expressions in vivo in the kidney of STZ-induced diabetic rats
Naofen mRNA expression significantly increased from baseline (control) obtained without STZ to 1.5 ± 0.11 at the 8th week (P < 0.05), to 2.44 ± 0.28 at the 16th week (P < 0.01), and further to 2.03 ± 0.16 at the 24th week (P < 0.01) (Fig. 2a).
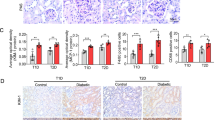
Naofen expression in kidney of streptozotocin (STZ)-induced diabetic and normal rats. Naofen mRNA expressions were measured by real-time PCR and are shown as values relative to GAPDH as an internal control (a). *P < 0.05 and **P < 0.01, compared with the control group, i.e., not treated with STZ. In situ hybridization for naofen was performed in the normal rats (b), and STZ-treated rats at the 16th and 24th weeks (c, d, respectively). Horizontal bar in d indicates 100 μm. Western blotting was performed with anti-naofen638–656 and anti-naofen1104–1120 peptide antibody, the same location of a band of 130 kDa was confirmed, and the amounts were calculated as ratios to GAPDH (e, f). **P < 0.01, compared with the control group. Experimental number was 6 in each group
In situ hybridization showed that naofen mRNA, in the control rats, was little observed and existed weakly in renal tubular epithelial cells and glomerular basement membrane (Fig. 2b), whereas in diabetic rats strong staining of naofen mRNA was obtained, especially in renal proximal/distal tubular epithelial cells in the preparations at the 16th and 24th weeks (Fig. 2c, d, respectively).
Western blot analysis of naofen
Western blot examination with anti-naofen1104–1120 antibody showed a band of naofen at 130 kDa in diabetic kidney, and similar results were obtained with anti-naofen638–656 antibody (Fig. 2e), while GAPDH (internal control protein) was demonstrated at 37 kDa. The amounts of naofen in the kidney of STZ-treated rats at the 16th and 24th weeks were significantly greater than those in the control (P < 0.01) (Fig. 2f).
Apoptosis in diabetic kidneys
Caspase-3 activities obtained at the 8th week were 1.5 times greater than those in the control group (P < 0.05), and 2.1 times at the 12th week (P < 0.01) (Fig. 3a).
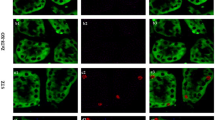
Relative caspase-3 activities (a), TUNEL staining (b–d), and number of TUNEL-positive cells per field (e). Caspase-3 activities were measured with fluorescence methods, relative values being shown as ratios to the control, i.e., not treated with streptozotocin (STZ), at each time point after STZ injection. *P < 0.05 and **P < 0.01, compared with the control group. Experimental number was 6 in each group. Results with TUNEL staining are shown for the normal rat kidney (b), and for STZ-induced diabetic rats at the 16th week after STZ injections (c) and at the 24th week (d). Arrows indicate TUNEL-positive cells. Horizontal bar indicates 100 μm. Number of TUNEL-positive cells per field at 100× magnification in renal tubular epithelial cells are shown in the control, and STZ-treated rats at the 16th and 24th weeks after STZ injections (e). ***P < 0.001, compared with normal rats
To assess whether apoptotic cell death may appear in diabetic kidney, tissue sections were labeled by in situ TUNEL assay (Fig. 3b–d). Apoptosis was obvious in both cortex and medulla of diabetic kidney: estimation of renal apoptosis revealed nearly eightfold increase in TUNEL-positive nuclei in diabetic kidneys compared with normal ones (Fig. 3e), and epithelial cells of both proximal and distal tubules were TUNEL positive.
Naofen mRNA expression in vitro in NRK52E cells
NRK52E cells were exposed to 5 mM (normal glucose, NG) or 25 mM (high glucose, HG) glucose, in the absence or presence of TNF-α (30 ng/ml) for 18 h. Naofen mRNA expression was significantly higher in the high glucose medium in either presence or absence of TNF-α in NRK52E cells (P < 0.05), no affected with TNF-α (Fig. 4a). These data suggest that naofen expression may be upregulated by high glucose environments. Also, mannitol (25 mM) did not affect naofen mRNA expression (data not shown), indicating that the results obtained with high glucose described above were not due to high osmotic pressure.
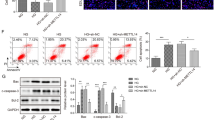
Naofen mRNA expression, and effect of naofen knockdown in caspase-3 activation in NRK52E cells. NRK52E cells were exposed to 5 mM (NG normal glucose level) or 25 mM (HG high glucose level) glucose, in the absence or presence of tumor necrosis factor (TNF)-α (30 ng/ml) for 18 h. Naofen mRNA expression was significantly higher in HG, regardless of TNF-α. *P < 0.05 compared with the NG group (a). After 24 h post transfection, NF-siRNA (10 nM) silenced naofen mRNA levels by 75%. **P < 0.01 and ## P < 0.01 compared with the without transfection (control) and transfection control-siRNA groups, respectively. Naofen mRNA values are shown as ratios to GAPDH (b). Treatment with HG significantly increased caspase-3 activities, and the increased caspase-3 activities were inhibited by transfection of NF-siRNA. *P < 0.05 compared with the NG group; # P < 0.05 compared with the HG group without transfection (c). Experimental number was 5–7
Effect of naofen-siRNA on naofen mRNA expression and caspase-3 activation
At 24 h after transfection with NF-siRNA (10 nM), cells were collected to detect expression of naofen mRNA by real-time PCR. Expression of naofen mRNA was significantly diminished by 75%, while control-siRNA elicited almost no effect on naofen mRNA expression (P < 0.01; Fig. 4b). These results indicate that naofen mRNA was effectively knocked down by transfection of siRNA in NRK52E cells.
To investigate caspase-3 activation induced by naofen knockdown, 24 h after transfection with NF-siRNA (10 nM), NRK52E cells were stimulated with normal or high glucose medium and cultured for another 18 h until the caspase-3 activity assay. Treatment with high glucose significantly increased caspase-3 activities in NRK52E cells (P < 0.05; Fig. 4c), and such an increase in caspase-3 activities was inhibited by transfection of NF-siRNA (P < 0.05). Treatment with normal glucose did not affect caspase-3 activities, with or without NF-siRNA treatment. Also, mannitol (25 mM) did not affect caspase-3 activity in NRK52E cells (data not shown). These data show that naofen may regulate caspase-3 activity in high-glucose-induced apoptosis.
Discussion
In STZ-induced diabetic kidney of rats, TUNEL staining and caspase activation showed apoptosis of proximal/distal tubular epithelial cells. Meanwhile, naofen mRNA expression was obviously induced, but not in liver (data not shown). Reportedly apoptosis in tubulo-interstitial cells may be closely correlated to fibrosis in kidney, resulting in renal dysfunction [15, 16]. Although TNF-α may be expressed in glomerular and proximal tubular cells in STZ-induced diabetic kidney, contributing to the pathogenesis of diabetic nephropathy [17–19], neither TNF-α (as shown in Fig. 4a), interleukin-1, nor interleukin-6 (data not shown) influenced naofen expression in the NRK52E cells, but high glucose enhanced it. These results indicate that naofen in NRK52E may be induced directly by high glucose, but probably not indirectly through inflammatory mediators such as TNF-α.
Western blotting performed with two kinds of anti-naofen antibodies (anti-naofen638–656 and anti-naofen1104–1120 antibodies) showed the same band of 130 kDa, almost equal to the calculated molecular weight of naofen, which consists of 1170 amino acids. In each antibody, naofen expression in STZ-induced diabetic kidney was increased, similar to the results obtained for mRNA. However it is still obscure how high glucose may enhance naofen expression.
Treatment of NRK52E cells with naofen siRNA clearly inhibited the increase in caspase-3 activity induced by incubation in high glucose medium. Such a result indicates that naofen might partly play a role in promoting caspase activity, especially in high-glucose-induced apoptosis of NRK52E cells. Ortiz et al. [20] and Kumar et al. [21] reported that STZ may cause apoptosis in kidney, especially in epithelial cells of both renal proximal and distal tubules. Many other investigators have also reported the possibility that hyperglycemia directly mediates apoptotic cell death in glomerular cells [22, 23], cultured endothelial cells [24], mesangium [25], and renal proximal tubules [26].
The blood glucose concentration obtained in STZ-induced diabetes of rats increased over time, leveling off 4 weeks after STZ injection, whereas the increase in naofen mRNA of kidney was also associated with glucose concentration, but the peak was obtained 16 weeks later. The caspase-3 activities were enhanced and leveled off 12 weeks after the injection, and the TUNEL staining positive cell population was also increased 24 weeks later. From the time courses of the in vivo studies, the increases in naofen expression and caspase activities were followed by morphological apoptotic changes. Reportedly, some factors, e.g., insulin-like growth factor-1 [27] and hepatocyte growth factor [28], may inhibit caspase-3 activity, preventing high-glucose-induced apoptosis. In such a context, the dissociation of peak times between caspase-3 (at the 12th week) and naofen (at the 16th week) may or may not be explained by these inhibitory factors. Furthermore, cell death through apoptosis has been documented in the course of renal injury in both animal models and clinical renal diseases [29, 30]. In diabetic nephropathy, tubular lesions such as the increases in apoptotic cell population related to tubular atrophy [31–33] might play a role in the development of renal functional changes in diabetes [34]. Thus, based on the results obtained in the present study, we hypothesize that naofen may elicit an important action in the development of apoptosis of tubular epithelial cells.
Noteworthy was that the TUNEL-positive cells observed in Fig. 3c, d were only sparse, while ISH with naofen probe showed much greater extent of both proximal and distal tubular epithelial cells. Caspase-3 activity in kidney of STZ-induced diabetic rats was increased twofold compared with in control rats. It was conceivable that TUNEL-positive cells may appear after caspase-3 activation through increased naofen expression.
In summary, the results obtained in the present in vivo and in vitro experiments suggest that naofen mRNA expression was increased under high glucose concentration in the plasma of STZ-treated rats, as well as in cultured NRK52E cells. Since knockdown of naofen inhibited caspase-3 activity and prevented high-glucose-induced apoptosis, naofen may be an important cytosolic protein which promotes apoptosis in diabetic kidney through hyperglycemia, especially in tubular epithelial cells. It is conceivable that naofen may be relevant to pathophysiological investigations.
References
Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–33.
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25.
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233:4–11.
Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39:497–504.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33.
Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16:705–13.
Nakao H, Takeda T. Escherichia coli Shiga toxin. J Nat Toxins. 2000;9:299–313.
Taguchi T, Uchida H, Kiyokawa N, Mori T, Sato N, Horie H, et al. Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int. 1998;53:1681–8.
Nakamura A, Johns EJ, Imaizumi A, Yanagawa Y, Kohsaka T. Activation of beta(2)-adrenoceptor prevents shiga toxin 2-induced TNF-alpha gene transcription. J Am Soc Nephrol. 2001;12:2288–99.
Yoshida T, Fukada M, Koide N, Ikeda H, Sugiyama T, Kato Y, et al. Primary cultures of human endothelial cells are susceptible to low doses of Shiga toxins and undergo apoptosis. J Infect Dis. 1999;180:2048–52.
An J, Feng GG, Huang L, Kurokawa T, Nonami T, Koide T, et al. Effects of 1-O-hexyl-2,3,5-trimethylhydroquinone on carbon tetrachloride-induced hepatic cirrhosis in rats. Hepatol Res (in press).
Feng GG, Yamada M, Wongsawatkul O, Li C, Huang L, An J, et al. Role of naofen, a novel WD repeat-containing protein, in reducing nitric oxide-induced relaxation. Clin Exp Pharmacol Physiol. 2008;35:1447–53.
Huang L, Hotta Y, Miyazeki K, Ishikawa N, Miki Y, Sugimoto Y, et al. Different effects of optical isomers of the 5-HT1A receptor antagonist pyrapyridolol against postischemic guinea-pig myocardial dysfunction and apoptosis through the mitochondrial permeability transition pore. Eur J Pharmacol. 2006;534:165–77.
Thomas GL, Yang B, Wagner BE, Savill J, El Nahas AM. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial Transplant. 1998;13:2216–26.
Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45.
Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia. 1999;42:878–86.
DiPetrillo K, Gesek FA. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am J Nephrol. 2004;24:352–9.
Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, et al. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450–6.
Ortiz A, Ziyadeh FN, Neilson EG. Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Investig Med. 1997;45:50–6.
Kumar D, Zimpelmann J, Robertson S, Burns KD. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:77–88.
Sugiyama H, Kashihara N, Makino H, Yamasaki Y, Ota A. Apoptosis in glomerular sclerosis. Kidney Int. 1996;49:103–11.
Savill J. Regulation of glomerular cell number by apoptosis. Kidney Int. 1999;56:1216–22.
Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhäusl W. High-glucose–triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–7.
Khera T, Martin J, Riley S, Steadman R, Phillips AO. Glucose enhances mesangial cell apoptosis. Lab Invest. 2006;86:566–77.
Ishii N, Ogawa Z, Suzuki K, Numakami K, Saruta T, Itoh H. Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism. 1996;45:1348–53.
Kang BP, Urbonas A, Baddoo A, Baskin S, Malhotra A, Meggs LG. IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am J Physiol Renal Physiol. 2003;285:1013–24.
Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Yamasaki K, et al. Hepatocyte growth factor prevents endothelial cell death through inhibition of bax translocation from cytosol to mitochondrial membrane. Diabetes. 2002;51:2604–11.
Ortiz A. Nephrology forum: apoptotic regulatory proteins in renal injury. Kidney Int. 2000;58:467–85.
Zhang W, Khanna P, Chan LL, Campbell G, Ansari NH. Diabetes-induced apoptosis in rat kidney. Biochem Mol Med. 1997;61:58–62.
Wang W, Tzanidis A, Divjak M, Thomson NM, Stein-Oakley AN. Altered signaling and regulatory mechanisms of apoptosis in focal and segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:1422–33.
Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, et al. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:173–80.
Tunçdemir M, Ozturk M. The effects of ACE inhibitor and angiotensin receptor blocker on clusterin and apoptosis in the kidney tissue of streptozotocin-diabetic rats. J Mol Histol. 2008;39:605–16.
Rasch R. Tubular lesions in streptozotocin-diabetic rats. Diabetologia. 1984;27:32–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10157-010-0287-y
About this article
Cite this article
Sato, Y., Feng, GG., Huang, L. et al. Enhanced expression of naofen in kidney of streptozotocin-induced diabetic rats: possible correlation to apoptosis of tubular epithelial cells. Clin Exp Nephrol 14, 205–212 (2010). https://doi.org/10.1007/s10157-010-0276-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-010-0276-1