Abstract
Background
Rectal cancer patients can conceivably obtain relief from neoadjuvant concurrent chemoradiotherapy (CCRT) for downstaging before resection, but the stratification of risk and clinical outcomes remains challenging. Therefore, identifying effective predictive biomarkers offers clinicians the opportunity to individually tailor early interventions, which would help optimize therapy.
Methods
Using a public rectal cancer transcriptome dataset (GSE35452), we focused on cytoskeletal protein binding (GO: 0008092)-related genes and identified FERM domain containing 3 (FRMD3) as the most significant differentially expressed gene associated with CCRT resistance. We gathered 172 tumor samples from rectal cancer patients treated with neoadjuvant CCRT accompanied by curative resection and estimated the expression level of FRMD3 using immunohistochemistry.
Results
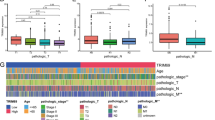
The results revealed that high FRMD3 immunoexpression was remarkably associated with advanced pre-CCRT and post-CCRT tumor status (p = 0.004 and p < 0.001), pre-CCRT and post-CCRT lymph node metastasis (both p < 0.001), more perineurial invasion (p = 0.023), and a smaller extent of tumor regression (p = 0.018). High FRMD3 immunoexpression was remarkably correlated with inferior disease-specific survival (DSS) (p = 0.0001), local recurrence-free survival (LRFS) (p = 0.0003), and metastasis-free survival (MeFS) (p = 0.0023) at the univariate level. Furthermore, in multivariate analysis, high FRMD3 immunoexpression remained independently predictive of inferior DSS (p = 0.002), LRFS (p = 0.005), and MeFS (p = 0.015).
Conclusion
These results suggest that high FRMD3 expression is related to advanced clinicopathological features and inferior therapeutic responses in rectal cancer patients treated with CCRT, validating the promising prognostic value of FRMD3 expression.



Similar content being viewed by others
Availability of data and materials
The dataset analyzed in the current study is available in the public transcriptome dataset GSE35452 from the GEO database (National Center for Biotechnology Information, Bethesda, MD, USA).
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30
Jemal A et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Fokas E et al (2014) Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 32(15):1554–1562
Cordes N, Meineke V (2003) Cell adhesion-mediated radioresistance (CAM-RR). Extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther Onkol 179(5):337–344
Landowski TH et al (2003) Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene 22(16):2417–2421
Dickreuter E, Cordes N (2017) The cancer cell adhesion resistome: mechanisms, targeting and translational approaches. Biol Chem 398(7):721–735
Ni X et al (2003) Molecular cloning and characterization of the protein 4.1O gene, a novel member of the protein 4.1 family with focal expression in ovary. J Hum Genet 48(2):101–106
Buffon MP et al (2015) FRMD3 gene: its role in diabetic kidney disease. A narrative review. Diabetol Metab Syndr 7:118
Nunomura W et al (1997) Regulation of CD44-protein 4.1 interaction by Ca2+ and calmodulin. Implications for modulation of CD44-ankyrin interaction. J Biol Chem 272(48):30322–30328
Haase D et al (2007) FRMD3, a novel putative tumour suppressor in NSCLC. Oncogene 26(30):4464–4468
Tran YK et al (1999) A novel member of the NF2/ERM/41 superfamily with growth suppressing properties in lung cancer. Cancer Res 59(1):35–43
Pezzolesi MG et al (2009) Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58(6):1403–1410
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12(1):19–23
Chan TC et al (2020) SLC14A1 prevents oncometabolite accumulation and recruits HDAC1 to transrepress oncometabolite genes in urothelial carcinoma. Theranostics 10(25):11775–11793
Kramer-Zucker AG et al (2005) Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285(2):316–329
Freedman BI et al (2011) Differential effects of MYH9 and APOL1 risk variants on FRMD3 Association with Diabetic ESRD in African Americans. PLoS Genet 7(6):e1002150
Al-waheeb S et al (2016) Evaluation of associations between single nucleotide polymorphisms in the FRMD3 and CARS genes and diabetic nephropathy in a Kuwaiti population. Genet Mol Res 15:114
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61(5):759–767
The Cancer Genome Atlas (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487(7407):330–7
O’Donnell KA et al (2005) c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435(7043):839–843
Watanabe T et al (2014) Prediction of response to preoperative chemoradiotherapy in rectal cancer by using reverse transcriptase polymerase chain reaction analysis of four genes. Dis Colon Rectum 57(1):23–31
Murphy JM et al (2020) Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp Mol Med 52(6):877–886
Frame MC et al (2010) The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol 11(11):802–814
Dickreuter E et al (2016) Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene 35(11):1353–1362
Vehlow A et al (2017) Adhesion- and stress-related adaptation of glioma radiochemoresistance is circumvented by β1 integrin/JNK co-targeting. Oncotarget 8(30):49224–49237
Meineke V et al (2002) Ionizing radiation modulates cell surface integrin expression and adhesion of COLO-320 cells to collagen and fibronectin in vitro. Strahlenther Onkol 178(12):709–714
Bloomgarden ZT (2005) Diabetic nephropathy. Diabetes Care 28(3):745–751
Eke I et al (2012) β1 Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest 122(4):1529–1540
Ou J et al (2012) αV integrin induces multicellular radioresistance in human nasopharyngeal carcinoma via activating SAPK/JNK pathway. PLoS ONE 7(6):e38737
Zeng Y et al (2015) Prognostic significance of interleukin-17 in solid tumors: a meta-analysis. Int J Clin Exp Med 8(7):10515–10536
Rokavec M et al (2014) IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest 124(4):1853–1867
Ferrao RD, Wallweber HJ, Lupardus PJ (2018) Receptor-mediated dimerization of JAK2 FERM domains is required for JAK2 activation. Elife 7:51
Du W et al (2012) Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J Cell Mol Med 16(8):1878–1888
Chang L et al (2016) Cancer stem cells and signaling pathways in radioresistance. Oncotarget 7(10):11002–11017
Giancotti FG (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155(4):750–764
Martini S et al (2013) From single nucleotide polymorphism to transcriptional mechanism: a model for FRMD3 in diabetic nephropathy. Diabetes 62(7):2605–2612
Fares J et al (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5(1):28
Zhou Y et al (2018) Cancer stem cells in progression of colorectal cancer. Oncotarget 9(70):33403–33415
Wang J et al (2010) Notch promotes radioresistance of glioma stem cells. Stem Cells 28(1):17–28
Bu P et al (2013) A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell 12(5):602–615
Yahyanejad S, Theys J, Vooijs M (2016) Targeting Notch to overcome radiation resistance. Oncotarget 7(7):7610–7628
Liu WH et al (2020) CD44-associated radioresistance of glioblastoma in irradiated brain areas with optimal tumor coverage. Cancer Med 9(1):350–360
Piao LS et al (2012) CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett 315(2):129–137
Dalerba P et al (2007) Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 104(24):10158–10163
Mori T et al (2008) Structural basis for CD44 recognition by ERM proteins. J Biol Chem 283(43):29602–29612
Lee H et al (2020) Prominin-1-Radixin axis controls hepatic gluconeogenesis by regulating PKA activity. EMBO Rep 21(11):e49416
Vishnubalaji R et al (2018) Molecular profiling of ALDH1(+) colorectal cancer stem cells reveals preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget 9(17):13551–13564
Park SY et al (2019) The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res 38(1):399
Author information
Authors and Affiliations
Contributions
Conceptualization: C-FL, H-YL; methodology: T-JC, C-LC, Y-FT, C-FY, T-CC, H-LH, W-SL, H-HT; investigation: T-JC, C-LC, Y-FT, C-FY, T-CC, H-LH, W-SL, H-HT; formal analysis: T-JC, C-LC, Y-FT, C-FY, T-CC, H-LH, W-SL, H-HT; resources: H-LH, W-SL, H-HT; validation: T-JC, C-LC, Y-FT, C-FY, T-CC; visualization: T-JC, C-LC, Y-FT, C-FY, T-CC; writing—original draft: C-FL, H-YL; writing—review and editing: C-FL, H-YL; funding acquisition: C-FL, H-YL; supervision: C-FL, H-YL. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
The study was approved by the Ethics Committee and Institutional Review Board of Chi Mei Medical Center (10302014) for the use of tumor samples disconnected from their identifiable information from the biobank following the ethical guidelines of the Helsinki Declaration and the regulations of our government.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Chen, TJ., Chou, CL., Tian, YF. et al. High FRMD3 expression is prognostic for worse survival in rectal cancer patients treated with CCRT. Int J Clin Oncol 26, 1689–1697 (2021). https://doi.org/10.1007/s10147-021-01944-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01944-6