Abstract
Background
Chemotherapy in relapsed colorectal cancer patients treated with oxaliplatin as adjuvant chemotherapy is under debate. REACT study aimed to investigate the efficacy of reintroducing modified FOLFOX6 (mFOLFOX6) or CAPOX with or without bevacizumab in recurrent colorectal cancer patients after oxaliplatin adjuvant chemotherapy.
Methods
Patients that participated in this trial had a medical history of adjuvant chemotherapy, including oxaliplatin with a cumulative dose greater than 400 mg/m2, and recurrence that was diagnosed more six months post adjuvant chemotherapy. Primary endpoints were response rate (RR) and disease control rate (DCR), while key secondary endpoints were time to treatment failure (TTF), progression-free survival (PFS), overall survival (OS), and safety.
Results
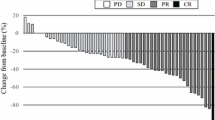
A total of 31 patients were enrolled between October 2012 and October 2016. Of the 29 eligible patients, 7 received mFOLFOX6 and 22 received CAPOX. The RR was 62.1% (95% confidence interval 42.3–79.3) and the DCR was 82.8% (95% confidence interval 64.2–94.2). The RR for oxaliplatin-free interval was 100.0% in months 6–12 and 56.0% after 12 months. Median TTF, PFS, and OS were 6.3, 10.8, and 28.7 months, respectively. Grade 3 or worse peripheral sensory neuropathy developed in 6.5%. Allergic reactions occurred in 12.9% of the patients, with one (3.2%) grade 3 episode. There were no other severe treatment-related adverse events.
Conclusion
Reintroduction of oxaliplatin was feasible and achieved high RR or DCR in patients after more than 6 months post oxaliplatin adjuvant chemotherapy.


Similar content being viewed by others
Abbreviations
- FOLFOX:
-
Levoleucovorin, 5-fluorouracil, and oxaliplatin
- CAPOX:
-
Capecitabine and oxaliplatin
- l-LV:
-
Levoleucovorin
- 5-FU:
-
5-Fluorouracil
- mFOLFOX6:
-
Modified FOLFOX6
- RR:
-
Response rate
- DCR:
-
Disease control rate
- TTF:
-
Time to treatment failure
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- PSN:
-
Peripheral sensory neuropathy
- mCRC:
-
Metastatic colorectal cancer
- RDI:
-
Relative dose intensity
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- NE:
-
Not evaluable
- RDI:
-
Relative dose intensity
- FAS:
-
Full analysis set
- CTCAE:
-
The Common Terminology Criteria for Adverse Events
- UMIN:
-
University Hospital Medical Information Network
- IDEA:
-
The International Duration Evaluation of Adjuvant Therapy
References
National Comprehensive Cancer Network(2019) NCCN Guidelines Version 3. 2019 Colon Cancer. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed Oct 2019
Yoshino T, Arnold D, Taniguchi H et al (2018) Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 29:44–70
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
André T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27:3109–3116
André T, de Gramont A, Vernerey D et al (2015) Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 33:4176–4187
Haller DG, Tabernero J, Maroun J et al (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29:1465–1471
Grothey A, Sobrero AF, Shields AF et al (2018) Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378:1177–1188
Sargent D, Shi Q, Yothers G et al (2011) Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 47:990–996
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol 24:394–400
Chibaudel B, Maindrault-Goebel F, Lledo G et al (2009) Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 27:5727–5733
de Gramont A, Buyse M, Abrahantes JC et al (2007) Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol 25:3224–3229
de Gramont A, Chibaudel B, Bourges O et al (2009) Definition of oxaliplatin sensitivity in patients with advanced colorectal cancer previously treated with oxaliplatin-based therapy. J Clin Oncol 27(Suppl 15):4024
Suenaga M, Mizunuma N, Matsusaka S et al (2015) Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE-OPEN study. Drug Des Dev Ther 9:3099–3108
Kotaka M, Yoshino T, Oba K et al (2015) Initial safety report on the tolerability of modified FOLFOX6 as adjuvant therapy in patients with curatively resected stage II or III colon cancer (JFMC41-1001-C2: JOIN trial). Cancer Chemother Pharmacol 76:75–84
Iveson TJ, Kerr RS, Saunders MP et al (2018) 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol 19:562–578
Sobrero A, Lonardi S, Rosati G et al (2018) FOLFOX or CAPOX in stage II to III colon cancer: efficacy results of the Italian three or six colon adjuvant trial. J Clin Oncol 36:1478–1485
André T, Vernerey D, Mineur L et al (2018) Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol 36:1469–1477
Souglakos J, Boukovinas I, Kakolyris S et al (2019) Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann Oncol 30:1304–1310
Yoshino T, Yamanaka T, Oki E et al (2019) Efficacy and long-term peripheral sensory neuropathy of 3 vs 6 months of oxaliplatin-based adjuvant chemotherapy for colon cancer: the ACHIEVE phase 3 randomized clinical trial. JAMA Oncol 5:1574–1581
Maindrault-Goebel F, Tournigand C, André T et al (2004) Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer. Ann Oncol 15:1210–1214
Chibaudel B, Tournigand C, Bonnetain F et al (2013) Platinum-sensitivity in metastatic colorectal cancer: towards a definition. Eur J Cancer 49:3813–3820
Saltz LB, Clarke S, Díaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Cassidy J, Clarke S, Díaz-Rubio E et al (2018) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Mori Y, Nishimura T, Kitano T et al (2010) Oxaliplatin-free interval as a risk factor for hypersensitivity reaction among colorectal cancer patients treated with FOLFOX. Oncology 79:136–143
Acknowledgements
We thank the patients, their families, and the investigators who participated in the REACT study.
Funding
This study was supported by Surgeons and Oncologists study group for developing New Strategy in Cancer Treatment (SONIC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Kotaka has received honoraria from Chugai Pharma and Yakult Honsha. S. Iwamoto and H. Satake have received honoraria from Chugai Pharma. D. Sakai has received honoraria and research funding from Chugai Pharma; research funding from Yakult Honsha. T. Kudo belong to a donated fund laboratory from Yakult Honsha, Chugai Pharma, and Ono Pharma. A. Tsuji has received honoraria from Chugai Pharma; research funding from Kyowa Kirin. N. Sugimoto has received research funding from Chugai Pharma. T. Satoh has received honoraria from Yakult Honsha; belonging to a donated fund laboratory from Yakult Honsha and Chugai Pharma. T. Yamanaka has received honoraria and research funding from Takeda Pharma, Chugai Pharma, Boehringer Ingelheim, Taiho pharma, Daiichi-Sankyo, and Ono Pharma; research funding from Merck Biopharma, Astellas Pharma, and Bayer. N. Tomita has received research funding from Chugai Pharma, Eli Lilly Japan, Taiho pharma, Beyer. and Sysmex. All remaining authors indicated no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kotaka, M., Iwamoto, S., Satake, H. et al. Evaluation of FOLFOX or CAPOX reintroduction with or without bevacizumab in relapsed colorectal cancer patients treated with oxaliplatin as adjuvant chemotherapy (REACT study). Int J Clin Oncol 25, 1515–1522 (2020). https://doi.org/10.1007/s10147-020-01701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01701-1