Abstract
Background
Anthracyclines are used to treat childhood acute lymphoblastic leukemia (ALL). Even when administered at low doses, these agents are reported to cause progressive cardiac dysfunction. We conducted a clinical trial comparing the toxicities of two anthracyclines, pirarubicin (THP) and daunorubicin (DNR), in the treatment of childhood ALL. The results from our study that relate to acute and late toxicities are reported here.
Methods
276 children with B-ALL were enrolled in the trial from April 1997 to March 2002 and were randomly assigned to receive a regimen including either THP (25 mg/m2 × 11) or DNR (30 mg/m2 × 11). Acute toxicity was prospectively assessed based on the National Cancer Institute Common Toxicity Criteria. Acute hematological toxicity was also examined via some parameters. Patients with event-free survival of >5 years were retrospectively surveyed for cardiac function at 5 and 10 years and at the most recent assessment more than 10 years from the onset of ALL.
Results
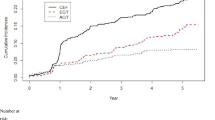
Acute hematological toxicity in the early phase was more significant in the THP arm. Based on ultrasound cardiography, cardiac function was impaired in both groups during the follow-up period, but there was no significant difference between the groups except for a greater decline in fractional shortening on ultrasound cardiography in the DNR arm.
Conclusions
While acute hematological toxicity was more significant in the THP arm, THP also appeared to be less cardiotoxic. However, the evaluation of late cardiotoxicity was limited because only a few subjects were followed beyond 10 years after ALL onset. Considering that the THP regimen produced an EFS rate comparable with that of the DNR regimen, the efficacy and toxicity of THP at reduced doses should be studied in order to identify potentially safer regimens.


Similar content being viewed by others
References
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373:1541–1552
Hitchcock-Bryan S, Gelber R, Cassady JR et al (1986) The impact of induction anthracycline on long-term failure-free survival in childhood acute lymphoblastic leukemia. Med Pediatr Oncol 14:211–215
Lipshultz SE, Lipsitz SR, Sallan SE et al (2005) Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23:2629–2636
Rathe M, Carlsen NL, Oxhøj H (2007) Late cardiac effects of anthracycline containing therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 48:663–667
Lipshultz SE, Giantris AL, Lipsitz SR et al (2002) Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 acute lymphoblastic leukemia protocol. J Clin Oncol 20:1677–1682
Russo D, Piccaluga PP, Michieli M et al (2002) Liposomal daunorubicin (DaunoXome) for treatment of poor-risk acute leukemia. Ann Hematol 81:462–466
Asselin BL, Devidas M, Chen L et al (2016) Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-Cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 34:854–862
Umezawa H, Takahashi Y, Kinoshita M et al (1979) Tetrahydropyranyl derivatives of daunomycin and adriamycin. J Antibiot (Tokyo) 32:1082–1084
Munck JN, Fourcade A, Bennoun M et al (1985) Relationship between the intracellular level and growth inhibition of a new anthracycline 4′O-tetrahydropyranyl-Adriamycin in Friend leukemia cell variants. Leuk Res 9:289–296
Tsuruo T, Iida H, Tsukagoshi S et al (1982) 4′-O-tetrahydropyranyladriamycin as a potential new antitumor agent. Cancer Res 42:1462–1467
Hirano S, Wakazono K, Agata N et al (1994) Comparison of cardiotoxicity of pirarubicin, epirubicin and doxorubicin in the rat. Drugs Exp Clin Res 20:153–160
Koh E, Ueda Y, Nakamura T et al (2002) Apoptosis in young rats with adriamycin-induced cardiomyopathy—comparison with pirarubicin, a new anthracycline derivative. Pediatr Res 51:256–259
Takagi T, Oguro M (1987) (2″-R)-4′-O-tetrahydropyranyladriamycin, a new anthracycline derivative; its effectiveness in lymphoid malignancies. Cancer Chemother Pharmacol 20:151–154
Hara J, Park YD, Yoshioka A et al (2001) Intensification of chemotherapy using block therapies as consolidation and reinduction therapies for acute lymphoblastic leukemia during childhood. Int J Hematol 74:165–172
Igarashi S, Manabe A, Ohara A et al (2005) No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Group L95-14 protocol. J Clin Oncol 23:6489–6498
Suzuki N, Yumura-Yagi K, Yoshida M et al (2010) Outcome of childhood acute lymphoblastic leukemia with induction failure treated by the Japan Association of Childhood Leukemia Study (JACLS) ALL F-protocol. Pediatr Blood Cancer 54(1):71–78
Tubergen DG, Gilchrist GS, O’Brien RT et al (1993) Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol 11:527–537
Harms DO, Janka-Schaub GE (2000) Co-operative Study Group for Childhood Acute Lymphoblastic Leukemia (COALL): long-term follow-up of trials 82, 85, 89 and 92. Leukemia 14:2234–2239
Gaynon PS, Steinherz PG, Bleyer WA et al (1993) Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Children’s Cancer Group Study CCG-106. J Clin Oncol 11:2234–2242
Nachman JB, Sather HN, Sensel MG et al (1998) Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med 338:1663–1671
Schrappe M, Reiter A, Zimmermann M et al (2000) Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Münster. Leukemia 14:2205–2222
Kaneko S, Tham EB, Haykowsky MJ et al (2016) Impaired left ventricular reserve in childhood cancer survivors treated with anthracycline therapy. Pediatr Blood Cancer 63:1086–1090
Armstrong GT, Ross JD (2014) Late cardiotoxicity in aging adult survivors of childhood cancer. Prog Pediatr Cardiol 36:19–26
Goorin AM, Chauvenet AR, Perez-Atayde AR et al (1990) Initial congestive heart failure, six to ten years after doxorubicin chemotherapy for childhood cancer. J Pediatr 116:144–147
Lipshultz SE, Colan SD, Gelber RD et al (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324:808–815
Taga T, Watanabe T, Tomizawa D et al (2016) Preserved high probability of overall survival with significant reduction of chemotherapy for myeloid leukemia in Down syndrome: a nationwide prospective study in Japan. Pediatr Blood Cancer 63:248–254
Takamatsu Y, Suzumiya J, Utsunomiya A et al (2010) THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol 84:391–397
Kudo K, Kojima S, Tabuchi K et al (2007) Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group. J Clin Oncol 25:5442–5447
Shimomura Y, Baba R, Watanabe A et al (2011) Assessment of late cardiotoxicity of pirarubicin (THP) in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 57:461–466
Feijen EA, Leisenring WM, Stratton KL et al (2015) Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol 33:3774–3780
Acknowledgements
We thank all of the patients who participated in this trial and all of the research staff at study centers who helped to recruit patients and provide data. This work was partly supported by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Hori, H., Kudoh, T., Nishimura, S. et al. Acute and late toxicities of pirarubicin in the treatment of childhood acute lymphoblastic leukemia: results from a clinical trial by the Japan Association of Childhood Leukemia Study. Int J Clin Oncol 22, 387–396 (2017). https://doi.org/10.1007/s10147-016-1062-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1062-1