Abstract
Background
Trim44 is an important member of the tripartite motif-containing protein (TRIM) family. Recent research reported that Trim44 might play an important role in tumorigenesis, although its role in non-small cell lung cancer (NSCLC) and the related mechanisms is not yet known.
Methods
In this study we analyzed 30 pairs of NSCLC tumors and the matched adjacent normal tissue to define the relationship between Trim44 and NSCLC tumors. The function of Trim44 in cell migration and invasion was determined by overexpression of Trim44 in normal bronchial epithelial cell line 16HE or knockdown of Trim44 in A549 cells, respectively. Whether Trim44-mediated NF-κB signaling activation was involved in Trim44-mediated promotion of lung cancer was tested by q-PCR analysis and cell migration and invasion assay using PDTC, an inhibitor of NF-κB.
Results
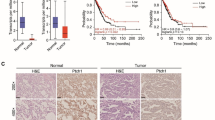
We found that Trim44 was upregulated in NSCLC tumors (14/30 cases; 46.7 %). Furthermore, Trim44 was upregulated in many NSCLC cell lines, especially in A549 and H441. Moreover, Trim44 significantly enhanced cell migration and invasion ability, which was related to increased CXCR6 and matrix metalloproteinase 9 (MMP9). Knockdown of Trim44 in A549 cells by siRNA showed a diminished effect in cell migration and invasion. Further investigation revealed that blocking the NF-κB signaling pathway using PDTC, an inhibitor of NF-κB, reversed the expression of CXCR6 and MMP9, and alleviated the promotion of migration and invasion mediated by Trim44.
Conclusions
Our data suggest that Trim44 promotes NSCLC development through activation of NF-κB signaling via upregulating CXCL16 and MMP9 expression.






Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics CA. Cancer J Clin 61:69–90
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Carthagena L, Bergamaschi A, Luna JM et al (2009) Human TRIM gene expression in response to interferons. PLoS One 4:e4894
McNab FW, Rajsbaum R, Stoye JP et al (2011) Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol 23:46–56
Ozato K, Shin DM, Chang TH et al (2008) TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 8:849–860
Jefferies C, Wynne C, Higgs R (2011) Antiviral TRIMs: friend or foe in autoimmune and autoinflammatory disease? Nat Rev Immunol 11:617–625
Li Q, Yan J, Mao AP et al (2011) Tripartite motif 8 (TRIM8) modulates TNFalpha- and IL-1beta-triggered NF-kappaB activation by targeting TAK1 for K63-linked polyubiquitination. Proc Natl Acad Sci USA 108:19341–19346
Nisole S, Stoye JP, Saib A (2005) TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol 3:799–808
Bieniasz PD (2004) Intrinsic immunity: a front-line defense against viral attack. Nat Immunol 5:1109–1115
Yang K, Shi HX, Liu XY et al (2009) TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol 182:3782–3792
Boutou E, Matsas R, Mamalaki A (2001) Isolation of a mouse brain cDNA expressed in developing neuroblasts and mature neurons. Brain Res Mol Brain Res 86:153–167
Yang B, Wang J, Wang Y (2013) Novel function of Trim44 promotes an antiviral response by stabilizing VISA. J Immunol 190:3613–3619
Peters CJ, Rees JR, Hardwick RH et al (2010) A 4-gene signature predicts survival of patients with resected adenocarcinoma of the esophagus, junction, and gastric cardia. Gastroenterology 139(e1915):1995–2004
Jarvinen AK, Autio R, Kilpinen S et al (2008) High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosom Cancer 47:500–509
Kashimoto K, Komatsu S, Ichikawa D et al (2012) Overexpression of TRIM44 contributes to malignant outcome in gastric carcinoma. Cancer Sci 103:2021–2026
Hu Y, Wang J, Yang B (2011) Guanylate binding protein 4 negatively regulates virus-induced type I IFN and antiviral response by targeting IFN regulatory factor 7. J Immunol 187:6456–6462
Beck G, Yard BA, Schulte J et al (2003) Secreted phospholipases A2 induce the expression of chemokines in microvascular endothelium. Biochem Biophys Res Commun 300:731–737
Chen L, Chen DT, Kurtyka C et al (2012) Tripartite motif containing 28 (Trim28) can regulate cell proliferation by bridging HDAC1/E2F interactions. J Biol Chem 287:40106–40118
Zhou ZY, Yang GY, Zhou J et al (2012) Significance of TRIM29 and beta-catenin expression in non-small-cell lung cancer. J Chin Med Assoc 75:269–274
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64:327–336
Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science (New York) 285:1028–1032
Stetler-Stevenson WG (1990) Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev 9:289–303
Zeng ZS, Cohen AM, Guillem JG (1999) Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis 20:749–755
Papathoma AS, Zoumpourlis V, Balmain A et al (2001) Role of matrix metalloproteinase-9 in progression of mouse skin carcinogenesis. Mol Carcinog 31:74–82
Cockett MI, Murphy G, Birch ML et al (1998) Matrix metalloproteinases and metastatic cancer. Biochem Soc Symp 63:295–313
Bianco FJ Jr, Gervasi DC, Tiguert R et al (1998) Matrix metalloproteinase-9 expression in bladder washes from bladder cancer patients predicts pathological stage and grade. Clin Cancer Res 4:3011–3016
Lee JM, Dedhar S, Kalluri R et al (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981
Tsuruta D (2009) NF-kappaB links keratinocytes and lymphocytes in the pathogenesis of psoriasis. Recent Pat Inflamm Allergy Drug Discov 3:40–48
Wang S, Uchi H, Hayashida S et al (2009) Differential expression of phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated p38 mitogen-activated protein kinase and nuclear factor-kappaB p105/p50 in chronic inflammatory skin diseases. J Dermatol 36:534–540
Acknowledgments
This work was supported by grants from the Shanghai Chest Hospital Science and Technology Fund Planning Project (YZ-11-28).
Conflict of interest
The authors have no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Q. Luo and H. Lin contributed equally to the work.
About this article
Cite this article
Luo, Q., Lin, H., Ye, X. et al. Trim44 facilitates the migration and invasion of human lung cancer cells via the NF-κB signaling pathway. Int J Clin Oncol 20, 508–517 (2015). https://doi.org/10.1007/s10147-014-0752-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0752-9