Abstract
Background
Breakthrough cancer pain typically has a rapid onset and relatively short duration. Due to this temporal profile, it may not be adequately relieved by oral opioid analgesics. The sublingual fentanyl orally disintegrating tablet is a formulation by which fentanyl can be rapidly absorbed across the oral mucosa producing rapid-onset analgesia, and which may be effective for breakthrough pain treatment.
Methods
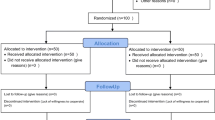
A multicenter, randomized, placebo-controlled, double-blind comparative study was conducted to evaluate the efficacy and safety of the sublingual fentanyl tablet at optimized doses for breakthrough pain treatment in cancer patients treated with strong opioid analgesics at fixed intervals. The optimal dose was determined by open-label dose titration. The efficacy and safety of a 12-week extended treatment were also evaluated.
Results
Eleven of 42 subjects who received the sublingual fentanyl tablet experienced adverse drug reactions. Common reactions were somnolence, constipation, nausea, and vomiting. No serious adverse reactions occurred. Sublingual fentanyl tablets at optimal doses and placebo were administered to 37 subjects in a double-blinded manner. A significant analgesic effect of the sublingual fentanyl tablet was present compared to placebo at 30 min after administration. The sublingual fentanyl tablet was also effective and safe during extended treatment, in which changes in basal opioid doses as well as sublingual fentanyl tablet doses were made as needed.
Conclusion
Sublingual fentanyl tablets at doses determined by titration were effective and safe for breakthrough pain treatment in cancer patients treated with strong opioid analgesics at fixed intervals. Extended treatment up to 12 weeks was also effective and safe.

Similar content being viewed by others
Notes
Abstral® is a registered trade name of Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan.
National Hospital Organization Hokkaido Cancer Center (Y Iwanami), Dokkyo Medical University Hospital (S Yamaguchi), Omigawa General Hospital (O Teramoto), Showa University Hospital (H Higuchi), Shizuoka Cancer Center (I Osaka), Shizuoka Saiseikai General Hospital (A Suga), National Hospital Organization Nagoya Medical Center (K Kondo), Saito Yukoukai Hospital (I Gomyo), Toyonaka Municipal Hospital (N Meguro), National Hospital Organization Kinki-Chuo Chest Medical Center (A Tokoro), Wakayama Rosai Hospital (Y Kobayashi), Hyogo College of Medicine Hospital (K Murakawa), Foundation for Biomedical Research and Innovation, Institute of Biomedical Research and Innovation Hospital (N Katakami), Shakaihoken Kobe Central Hospital (M Okada), Shimane University Hospital (Y Saito), Nagasaki University Hospital (M Hojo), Kumamoto University Hospital (H Kohrogi), Garatia Hospital (S Kanemura), National Center for Geriatrics and Gerontology (K Nakashima), Ibaraki Prefectural Central Hospital (S Mitsuhashi), Gifu Municipal Hospital (T Ishiguro), Matsue City Hospital (T Iwashita).
References
Potenoy RK, Hagen NA (1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41:273–281
Simmonds MA (1999) Management of breakthrough pain due to cancer. Oncology (Williston Park) 13:1103–1108
Bennett D, Burton AW, Fishman S et al (2005) Consensus panel recommendations for the assessment and management of breakthrough pain: Part 2. Management. Pharmacol Ther 30:354–361
Actiq SPC (2012) Cephalon Ltd, UK
Bredenberg S, Duberg M, Lennernas B et al (2003) In vitro and in vivo evaluation of a new sublingual tablet system for rapid oromucosal absorption using fentanyl citrate as the active substance. Eur J Pharm Sci 20:327–334
Streisand JB, Busch MA, Egan TD et al (1998) Dose proportionality and pharmacokinetics of oral transmucosal fentanyl citrate. Anesthesiology 88:305–309
Lennernas B, Hedner T, Holmberg M et al (2004) Pharmacokinetics and tolerability of different doses of fentanyl following sublingual administration of a rapidly dissolving tablet to cancer patients: a new approach to treatment of incident pain. Br J Clin Pharmacol 59:249–253
England R, Maddocks M, Manderson C et al (2011) How practical are transmucosal fentanyl products for breakthrough cancer pain? novel use of placebo formulations to survey user opinion. BMJ Support Palliat Care 1:349–351
Christie JM, Simmonds M, Patt R et al (1998) Dose-titration, multicenter study of oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients using transdermal fentanyl for persistent pain. J Clin Oncol 16:3238–3245
Payne R, Coluzzi P, Hart L et al (2011) Long-term safety of oral transmucosal fentanyl citrate for breakthrough cancer pain. J Pain Symptom Manag 22:575–583
Cherny NI, Portenoy RK (1993) Cancer pain management: current strategy. Cancer 72(Suppl 11):3393–3415
Yamaguchi T, Shima Y, Morita T et al (2013) Clinical guideline for pharmacological management of cancer pain: the Japanese society of palliative medicine recommendations. Jpn J Clin Oncol 43:896–909
Rauck RL, Tark M, Reyes E et al (2009) Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin 25:2877–2885
Abstral SPC (2013) ProStrakan Ltd, Galashiels, UK
Abstral SPC (2013) Galena Biopharma, Inc., Lake Oswego, US
Todd KH, Funk JP (1996) The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med 3:142–146
Acknowledgments
The sponsor of this clinical trial was Kyowa Hakko Kirin, Co., Ltd.
Conflict of interest
During the past 3 years, Naohito Shimoyama has had a contract as a medical expert with Kyowa Hakko Kirin Co., Ltd., Nippon Kayaku Co., Ltd. and Mitsubishi Tanabe Pharma Corporation. Naohito Shimoyama also has had a contract with Teikoku Seiyaku Co., Ltd. as a coordinating investigator and a member of independent data monitoring committee. Nobuyuki Yukitoshi and Eri Ohta are employees of Kyowa Hakko Kirin Co., Ltd. The other authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trials Government; NCT01326689.
About this article
Cite this article
Shimoyama, N., Gomyo, I., Katakami, N. et al. Efficacy and safety of sublingual fentanyl orally disintegrating tablet at doses determined by titration for the treatment of breakthrough pain in Japanese cancer patients: a multicenter, randomized, placebo-controlled, double-blind phase III trial. Int J Clin Oncol 20, 198–206 (2015). https://doi.org/10.1007/s10147-014-0697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0697-z