Abstract
Background
Oral uracil-tegafur and leucovorin (UFT/LV) therapy for elderly patients with metastatic colorectal cancer (mCRC) requires careful handling in Western countries because of a high incidence (≥20 %) of grade 3 diarrhea. However, its efficacy and safety for elderly Asian patients have not been investigated.
Methods
In this multicenter cooperative phase II study, the eligibility criteria were: age of 75 years or older, no prior chemotherapy, and histologically confirmed colorectal cancer with one or more measurable lesions. UFT 300 mg/m2/day and LV 75 mg/day were administered orally for 28 days followed by a 7-day rest period.
Results
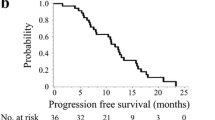
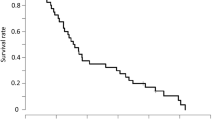
Twenty-one patients were enrolled in this study (prior to study termination after approval of bevacizumab), and all patients were eligible for efficacy and safety analysis. The median age was 79 years (range, 75–83 years). The majority of patients (95 %) had ECOG Performance Status 0 or 1. The overall response rate was 33 % (95 % confidence interval [CI], 18–53 %). The median progression-free and overall survivals were 5.3 months (95 % CI 4.0–7.9 months) and 18 months (95 % CI 13–21 months), respectively. Grade 3 or greater adverse events included anorexia (10 %), diarrhea (10 %), and leukopenia (5 %). These results were compatible with those seen in Japanese patients in a previous bridging study between Japan and the US, in which patients under 75 years old were evaluated.
Conclusions
UFT/LV therapy was safe and feasible in elderly Japanese patients with mCRC, and further study of UFT/LV therapy in combination with bevacizumab is warranted.

Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Gallego R, Sanchez N, Maurel J (2006) Chemotherapy for elderly patients with advanced colorectal carcinoma. Expert Rev Anticancer Ther 6:795–800
Van Cutsem E, Nordlinger B, Cervantes A (2010) Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol 21:93–97
National Comprehensive Cancer Network (2012) NCCN guidelines for: colon cancer (version 3.2012). http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 2012
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Douillard JY, Hoff PM, Skillings JR et al (2002) Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20:3605–3616
Carmichael J, Popiela T, Radstone D et al (2002) Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20:3617–3627
Shirao K, Hoff PM, Ohtsu A et al (2004) Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV. J Clin Oncol 22:3466–3474
Diaz-Rubio E, Sastre J, Abad A, et al (1999) UFT plus or minus calcium folinate for metastatic colorectal cancer in older patients. Oncology 13(suppl. 3):35–40
Hochster HS, Luo W, Elizabeta C et al (2007) Phase II study of uracil-tegafur with leucovorin in elderly (≥75 years old) patients with colorectal cancer: ECOG 1299. J Clin Oncol 25:3523–3529
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Price TJ, Zannino D, Wilson K (2012) Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the AGITG MAX trial: an international randomised controlled trial of Capecitabine, Bevacizumab and Mitomycin C. Ann Oncol 23:1531–1536
Seymour MT, Thompson CL, Wasan H et al (2011) Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 377:1749–1759
Peuguniez C, Fouriner C, Guerin-Meyer C et al (2011) A randomized cross-over trial comparing single-agents Capecitabine (C) and UFT plus leucovorin (LV) in patients with advanced colorectal cancer (CRC): final analysis of a patients preference study. J Clin Oncol 29 suppl 4;abstr 558
Hyodo I, Shirao K, Doi T et al (2006) A phase II study of the global dose and schedule of capecitabine in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 6(7):410–417
Kumar R, Jain K, Beeke C et al (2013) A population-based study of metastatic colorectal cancer in individuals aged ≥80 years. Cancer 119:722–728
Cunningham D, Lang I, Marcuello E et al (2013) Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 14:1077–1085
Puthillath A, Mashtare T Jr, Wilding G et al (2009) A phase II study of first-line biweekly capecitabine and bevacizumab in elderly patients with metastatic colorectal cancer. Crit Rev Oncol/Hematol 71:242–248
Feliu J, Safont J, Salud A et al (2010) Capecitabine and bevacizumab as first-line treatment in elderly patients with metastatic colorectal cancer. Br J Cancer 102:1468–1473
Françoisa E, Guérinb O, Follana P et al (2011) Use of bevacizumab in elderly patients with metastatic colorectal cancer: review. J Geriatr Oncol 2:64–71
Acknowledgments
We thank all of the participating patients, their family members, and the researchers. We are deeply grateful to Dr. K. Araki and Dr. N. Kobayashi for helping this study as the safety monitoring committee members.
Conflict of interest
Ichinosuke Hyodo and Toshikazu Moriwaki received a research grant from Taiho Pharmaceutical Co. Ltd.; Tomohiro Nishina, Minoru Mizuta, Akihito Tsuji, Ryohei Watanabe, Ikuo Takahashi, Yuji Watanabe, Toshikazu Moriwaki, Takashi Maeba and Ichinosuke Hyodo received lecture fees from Taiho Pharmaceutical Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Matsumoto, T., Nishina, T., Mizuta, M. et al. Phase II study of first-line chemotherapy with uracil-tegafur plus oral leucovorin in elderly (≥75 years) Japanese patients with metastatic colorectal cancer: SGOSG-CR0501 study. Int J Clin Oncol 20, 111–116 (2015). https://doi.org/10.1007/s10147-014-0675-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0675-5