Abstract
Background
Five randomized trials of adjuvant trastuzumab have reported significant improvements in recurrence-free survival (RFS) and overall survival. However, patients with node-negative tumors 1 cm or smaller were excluded from these trials. We assessed the recurrence risk and benefit of adjuvant therapy in such patients with small tumors.
Methods
We identified patients with node-negative breast tumors 1 cm or smaller between April 2003 and December 2007. Patients were categorized according to HER2 status and pathological tumor size (pT <5 mm vs. 5–10 mm), hormone receptor (HR) status and adjuvant chemotherapy. The primary endpoint was RFS.
Results
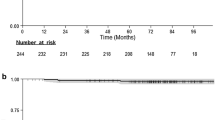
Of 267 patients included in the analysis, 42 had HER2-positive tumors. The median follow-up was 4.3 years. RFS was worse in patients with HER2-positive tumors than HER2-negative tumors (90.5 vs. 97.7% at 5 years; P = 0.031). In the group with HER2-positive tumors, there were no recurrences in patients with pT<5 mm, but 4 recurrences in those with pT 5–10 mm. RFS was worse in patients with pT 5–10 mm than pT <5 mm (79.0 vs. 100%, P = 0.025). Furthermore 3 recurrences occurred in patients without adjuvant trastuzumab, and 1 recurrence occurred as soon as adjuvant trastuzumab was finished. Our results appear to establish the efficacy of adjuvant trastuzumab therapy. HR status and use of adjuvant chemotherapy were not significantly associated with RFS.
Conclusions
Patients with HER2-positive, node-negative breast tumors 1 cm or smaller (especially 0.5–1.0 cm) have a significant recurrence risk and the decision to employ adjuvant trastuzumab therapy should be discussed with patients based on our results and those of other studies.





Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Slamon DJ, Godolphin W, Jones LA et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Burstein HJ (2005) The distinctive nature of HER2-positive breast cancers. N Engl J Med 353:1652–1654
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Slamon DEW, Robert N, Pienkowski T et al (2009) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients : BCIRG 006 study. Cancer Res 69(suppl. 24):500s (abstr 62)
Joensuu H, Kellokumpu-Lehtinen PL, Bono P et al (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809–820
Gonzalez-Angulo AM, Litton JK, Broglio KR et al (2009) High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 27:5700–5706
Curigliano G, Viale G, Bagnardi V et al (2009) Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 27:5693–5699
McArthur HL MK, Morris PG, Patil S et al (2010) Use of adjuvant trastuzumab with chemotherapy in women with small, node negative, HER2-positive breast cancers. J Clin Oncol 28(suppl.):15s (abstr615)
Rodrigues MJ, Wassermann J, Albiges L et al (2010) Trastuzumab treatment in t1ab, node-negative, human epidermal growth factor receptor 2-overexpressing breast carcinomas. J Clin Oncol 28:e541–e542
Tan-Chiu E, Yothers G, Romond E et al (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23:7811–7819
Perez EA, Suman VJ, Davidson NE et al (2008) Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 26:1231–1238
Telli ML, Hunt SA, Carlson RW et al (2007) Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25:3525–3533
Cardinale D, Colombo A, Torrisi R et al (2010) Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 28:3910–3916
Suter TM, Procter M, van Veldhuisen DJ et al (2007) Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 25:3859–3865
National Institute for Health and Clinical Excellence (2006) Trastuzumab for the adjuvant treatment of early-stage HER2 positive breast cancer. http://www.nice.org.uk/TA107
Purmonen TT, Pankalainen E, Turunen JH et al (2011) Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: cost-effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer Trial. Acta Oncol 50:344–352
Conflict of interest
H. Iwata received fees for lectures from Chugai Pharmaceutical. Other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Horio, A., Fujita, T., Hayashi, H. et al. High recurrence risk and use of adjuvant trastuzumab in patients with small, HER2-positive, node-negative breast cancers. Int J Clin Oncol 17, 131–136 (2012). https://doi.org/10.1007/s10147-011-0269-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0269-4