Abstract
Background. Some trials have suggested that the combination of gemcitabine and platinum compounds can have a synergistic effect on several solid tumors, but, at present, the data concerning carboplatin-gemcitabine combinations are not sufficient to allow the planning of phase II trials. The present phase I trial was planned to define the maximum tolerated dose and the dose-limiting toxicity of a carboplatin-gemcitabine combination.
Methods. Thirty-two patients with advanced, pretreated solid tumors were treated with carboplatin on day 1 and gemcitabine on days 1, 8, and 15 every 28 days. The starting doses of carboplatin and gemcitabine were 3.5 mg/ml per min (area under the curve; AUC), and 600 mg/m2, respectively. The doses of the two agents were alternately increased to 4, 4.5, and 5 mg/ml per min and to 800 and 960 mg/m2, respectively. At each dose level, three patients were initially enrolled. If one of them experienced grade IV hematological toxicity or grade III–IV nonhematological toxicity (with the exception of alopecia), an additional three patients were enrolled at the same dose level. If two or more patients experienced grade IV hematological toxicity or grade III–IV non-hematological toxicity (with the exception of alopecia), the maximum tolerated dose was considered to have been reached, and the dose below this was recommended for further studies. All patients were evaluated weekly for toxicity and after every two courses of chemotherapy for response.
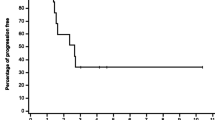
Results. Dose-limiting toxicity was hematological, and the maximum tolerated doses were 4.5 mg/ml per min for carboplatin and 800 mg/m2 for gemcitabine. The activity of the carboplatin/gemcitabine combination was encouraging, with a 21.9% response rate (7/32), three complete disease regressions, and a median time to progression of 30 weeks. The gemcitabine doses of day 15 or days 8 and 15 were omitted for hematological toxicity in 57 (50%) and 17 (14.9%) courses of chemotherapy, while no courses of chemotherapy were delayed for grade III–IV hematological or nonhematological toxicity.
Conclusion. The maximum tolerated doses suggested by this trial are lower than those in other similar phase I trials, but they are consistent with those reported by most of the trials investigating gemcitabine either in combination with cisplatin or in heavily pretreated patients. Carboplatin 4.5 mg/ml per min on day 1 plus gemcitabine 800 mg/m2 on days 1, 8, and 15 every 28 days may represent a promising schedule for further phase II trials.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: January 29, 2001 / Accepted: September 13, 2001
About this article
Cite this article
Tassinari, D., Drudi, G., Panzini, I. et al. Combination chemotherapy of carboplatin and gemcitabine against solid tumors: a phase I trial. Int J Clin Oncol 6, 279–283 (2001). https://doi.org/10.1007/s10147-001-8028-6
Issue Date:
DOI: https://doi.org/10.1007/s10147-001-8028-6