Abstract
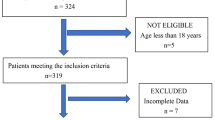
Growing evidence has suggested that hyperhomocysteinemia (HHcy) is a risk factor for cerebral infarction. However, the effect of HHcy on postoperative cerebral ischemia is still unclear. We aim to investigate the relationship between HHcy and postoperative ischemia of adult patients with moyamoya disease (MMD). A total of 138 adult patients with MMD were prospectively recruited from July 1 to December 31, 2019. After excluding 14 patients accepting conservative therapy, all 124 patients who underwent surgical treatment were enrolled. Patients were grouped according to postoperative ischemia and HHcy presentation, respectively. Clinical data and laboratory examinations were compared by statistical analyses. Potential risk factors were evaluated by univariate and multivariate logistic regression analysis. Comparing to the normal, patients with postoperative ischemia were higher in serum homocysteine (Hcy) level (P = 0.039) and HHcy ratio (P = 0.035). Furthermore, HHcy was more common in males (P = 0.007) than females. Logistic analysis results showed that HHcy (OR 5.234, 95% CI 1.127–24.315; P = 0.035) was an independent risk factor. HHcy was significantly associated with postoperative ischemia in MMD patients. Our study found that HHcy was correlated to the risk of postoperative ischemia. HHcy can be used as an indicator and a potential therapeutic target for postoperative ischemia in adult patients with MMD. URL: http://www.chictr.org. Unique identifier: ChiCTR2000031412.
Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Acker G, Fekonja L, Vajkoczy P (2018) Surgical management of moyamoya disease. Stroke 49:476–482. https://doi.org/10.1161/strokeaha.117.018563
Ando T, Shimada Y, Fujiwara S, Yoshida K, Kobayashi M, Kubo Y, Terasaki K, Ando S, Ogasawara K (2020) Revascularisation surgery improves cognition in adult patients with moyamoya disease. J Neurol Neurosurg Psychiatry 91:332–334. https://doi.org/10.1136/jnnp-2019-321069
Anniwaer J, Liu M, Xue K, Maimaiti A, Xiamixiding A (2019) Homocysteine might increase the risk of recurrence in patients presenting with primary cerebral infarction. Int J Neurosci 129:654–659. https://doi.org/10.1080/00207454.2018.1517762
Bang OY, Fujimura M, Kim SK (2016) The pathophysiology of moyamoya disease: an update. J Stroke 18:12–20. https://doi.org/10.5853/jos.2015.01760
Cho H, Jo K, Yu J, Yeon J, Hong S, Kim J (2017) Low flow velocity in the middle cerebral artery predicting infarction after bypass surgery in adult moyamoya disease. J Neurosurg 126:1573–1577. https://doi.org/10.3171/2016.3.jns152256
Coppola A, Davi G, De Stefano V, Mancini F, Cerbone A, Di Minno G (2000) Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost 26:243–254. https://doi.org/10.1055/s-2000-8469
Deep SN, Mitra S, Rajagopal S, Paul S, Poddar R (2019) GluN2A-NMDA receptor-mediated sustained Ca(2+) influx leads to homocysteine-induced neuronal cell death. J Biol Chem 294:11154–11165. https://doi.org/10.1074/jbc.RA119.008820
Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S, Cao Y, Zhao Y, Pan Y, Liu X, Zhang Q, Zhao J (2018) Direct versus indirect bypasses for adult ischemic-type moyamoya disease: a propensity score-matched analysis. J Neurosurg 128:1785–1791. https://doi.org/10.3171/2017.2.jns162405
Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S, Cao Y, Zhao Y, Pan Y, Ye X, Liu X, Zhang Q, Wang J, Yang Z, Zhao M, Zhao J (2018) Effects of different surgical modalities on the clinical outcome of patients with moyamoya disease: a prospective cohort study. J Neurosurg 128:1327–1337. https://doi.org/10.3171/2016.12.jns162626
Fan H, Yang S, Li Y, Yin J, Qin W, Yang L, Yuan J, Hu W (2018) Assessment of homocysteine as a diagnostic and early prognostic biomarker for patients with acute lacunar infarction. Eur Neurol 79:54–62. https://doi.org/10.1159/000484893
Ge P, Zhang Q, Ye X, Liu X, Deng X, Wang J, Wang R, Zhang Y, Zhang D, Zhao J (2020) Modifiable risk factors associated with moyamoya disease: a case-control study. Stroke 51:2472–2479. https://doi.org/10.1161/strokeaha.120.030027
Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis) (2012). Neurologia medico-chirurgica 52:245-266. https://doi.org/10.2176/nmc.52.245
Hankey G, Eikelboom J (2001) Homocysteine and stroke. Curr Opin Neurol 14:95–102. https://doi.org/10.1097/00019052-200102000-00015
Holmes M, Newcombe P, Hubacek J, Sofat R, Ricketts S, Cooper J, Breteler M, Bautista L, Sharma P, Whittaker J, Smeeth L, Fowkes F, Algra A, Shmeleva V, Szolnoki Z, Roest M, Linnebank M, Zacho J, Nalls M, Singleton A, Ferrucci L, Hardy J, Worrall B, Rich S, Matarin M, Norman P, Flicker L, Almeida O, van Bockxmeer F, Shimokata H, Khaw K, Wareham N, Bobak M, Sterne J, Smith G, Talmud P, van Duijn C, Humphries S, Price J, Ebrahim S, Lawlor D, Hankey G, Meschia J, Sandhu M, Hingorani A, Casas J (2011) Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet (London, England) 378:584–594. https://doi.org/10.1016/s0140-6736(11)60872-6
Kaplan P, Tatarkova Z, Sivonova M, Racay P, Lehotsky J (2020) Homocysteine and mitochondria in cardiovascular and cerebrovascular systems. Int J Mol Sci 21. https://doi.org/10.3390/ijms21207698
Kim J, Jeon J (2014) An update on the diagnosis and treatment of adult Moyamoya disease taking into consideration controversial issues. Neurol Res 36:407–416. https://doi.org/10.1179/1743132814y.0000000351
Kim T, Oh C, Bang J, Kim J, Cho W (2016) Moyamoya disease: treatment and outcomes. J Stroke 18:21–30. https://doi.org/10.5853/jos.2015.01739
Kraemer M, Berlit P, Diesner F, Khan N (2012) What is the expert’s option on antiplatelet therapy in moyamoya disease? Results of a worldwide survey. Eur J Neurol 19:163–167. https://doi.org/10.1111/j.1468-1331.2011.03481.x
Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, Tsuji I, Inaba Y, Yoshimoto T (2008) Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke 39:42–47. https://doi.org/10.1161/strokeaha.107.490714
Kuroda S, Houkin K (2008) Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7:1056–1066. https://doi.org/10.1016/s1474-4422(08)70240-0
Lai W, Kan M (2015) Homocysteine-induced endothelial dysfunction. Ann Nutr Metab 67:1–12. https://doi.org/10.1159/000437098
Ma Y, Li L, Geng X, Hong Y, Shang X, Tan Z, Song Y, Zhao G, Zhao B, Tian M (2016) Correlation between hyperhomocysteinemia and outcomes of patients with acute myocardial infarction. Am J Ther 23:e1464–e1468. https://doi.org/10.1097/mjt.0000000000000130
Moll S, Varga E (2015) Homocysteine and MTHFR mutations. Circulation 132:e6–e9. https://doi.org/10.1161/circulationaha.114.013311
Mukerji N, Cook D, Steinberg G (2015) Is local hypoperfusion the reason for transient neurological deficits after STA-MCA bypass for moyamoya disease? J Neurosurg 122:90–94. https://doi.org/10.3171/2014.8.jns132413
Oki K, Katsumata M, Izawa Y, Takahashi S, Suzuki N, Houkin K (2018) Trends of antiplatelet therapy for the management of moyamoya disease in Japan: results of a nationwide survey. J Stroke Cerebrovasc Dis 27:3605–3612. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.08.030
Pandey P, Steinberg G (2011) Neurosurgical advances in the treatment of moyamoya disease. Stroke 42:3304–3310. https://doi.org/10.1161/strokeaha.110.598565
Peng Y, Huang M, Xue Y, Pan J, Lin C (2020) Association of hyperhomocysteinemia with increased coronary microcirculatory resistance and poor short-term prognosis of patients with acute myocardial infarction after elective percutaneous coronary intervention. Biomed Res Int 2020:1710452–1710458. https://doi.org/10.1155/2020/1710452
Pezzini A, Del Zotto E, Padovani A (2007) Homocysteine and cerebral ischemia: pathogenic and therapeutical implications. Curr Med Chem 14:249–263. https://doi.org/10.2174/092986707779941140
Poddar R, Paul S (2009) Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death. J Neurochem 110:1095–1106. https://doi.org/10.1111/j.1471-4159.2009.06207.x
Qian Y, Huang B, Hu Z, Wang J, Zhao P, Li X (2020) Analysis of factors related to cerebral infarction after direct bypass surgery in adults with moyamoya disease. Cerebrovasc Dis (Basel, Switzerland) 49:55–61. https://doi.org/10.1159/000504743
Sato K, Morofuji Y, Horie N, Izumo T, Anda T, Matsuo T (2020) Hyperhomocysteinemia causes severe intraoperative thrombotic tendency in superficial temporal artery-middle cerebral artery bypass. J Stroke Cerebrovasc Dis 29:104633. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104633
Scott R, Smith E (2009) Moyamoya disease and moyamoya syndrome. N Engl J Med 360:1226–1237. https://doi.org/10.1056/NEJMra0804622
Scott RM, Smith ER (2009) Moyamoya disease and moyamoya syndrome. N Engl J Med 360:1226–1237. https://doi.org/10.1056/NEJMra0804622
Spence J (2007) Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol 6:830–838. https://doi.org/10.1016/s1474-4422(07)70219-3
Suzuki J, Takaku A (1969) Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20:288–299. https://doi.org/10.1001/archneur.1969.00480090076012
Wu W, Guan Y, Xu K, Fu X, Lei X, Lei L, Zhang Z, Cheng Y, Li Y (2016) Plasma homocysteine levels predict the risk of acute cerebral infarction in patients with carotid artery lesions. Mol Neurobiol 53:2510–2517. https://doi.org/10.1007/s12035-015-9226-y
Yamada S, Oki K, Itoh Y, Kuroda S, Houkin K, Tominaga T, Miyamoto S, Hashimoto N, Suzuki N (2016) Effects of surgery and antiplatelet therapy in ten-year follow-up from the Registry Study of Research Committee on Moyamoya Disease in Japan. J Stroke Cerebrovasc Dis 25:340–349. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.10.003
Yang Z, Wang L, Zhang W, Wang X, Zhou S (2016) Plasma homocysteine involved in methylation and expression of thrombomodulin in cerebral infarction. Biochem Biophys Res Commun 473:1218–1222. https://doi.org/10.1016/j.bbrc.2016.04.042
Yu L, Ma L, Huang Z, Shi Z, Wang R, Zhao Y, Zhang D (2019) Revascularization surgery in patients with ischemic-type moyamoya disease: predictors for postoperative stroke and long-term outcomes. World Neurosurg 128:e582–e596. https://doi.org/10.1016/j.wneu.2019.04.214
Yuan X, Wang T, Gao J, Wang Y, Chen Y, Kaliannan K, Li X, Xiao J, Ma T, Zhang L, Shao Z (2020) Associations of homocysteine status and homocysteine metabolism enzyme polymorphisms with hypertension and dyslipidemia in a Chinese hypertensive population. Clin Exp Hyperten (New York, NY : 1993) 42:52–60. https://doi.org/10.1080/10641963.2019.1571599
Zaric B, Obradovic M, Bajic V, Haidara M, Jovanovic M, Isenovic E (2019) Homocysteine and hyperhomocysteinaemia. Curr Med Chem 26:2948–2961. https://doi.org/10.2174/0929867325666180313105949
Zhang J, Li S, Fujimura M, Lau T, Wu X, Hu M, Zheng H, Xu H, Zhao W, Li X, Chen J (2019) Hemodynamic analysis of the recipient parasylvian cortical arteries for predicting postoperative hyperperfusion during STA-MCA bypass in adult patients with moyamoya disease. J Neurosurg 134:1–8. https://doi.org/10.3171/2019.10.jns191207
Zhao M, Deng X, Zhang D, Wang S, Zhang Y, Wang R, Zhao J (2018) Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J Neurosurg 130:1–12. https://doi.org/10.3171/2017.10.jns171749
Zhao Y, Lu J, Yu S, Li J, Deng X, Zhang Y, Zhang D, Wang R, Wang H, Zhao Y (2019) Comparison of long-term effect between direct and indirect bypass for pediatric ischemic-type moyamoya disease: a propensity score-matched study. Front Neurol 10:795. https://doi.org/10.3389/fneur.2019.00795
Zhao Y, Yu S, Lu J, Yu L, Li J, Zhang Y, Zhang D, Wang R, Zhao Y (2018) Direct bypass surgery vs. combined bypass surgery for hemorrhagic moyamoya disease: a comparison of angiographic outcomes. Front Neurol 9:1121. https://doi.org/10.3389/fneur.2018.01121
Consent for publication
All authors have read and approved the final manuscript.
Code availability
The SPSS software (version 26.0) was used for all the statistical analyses.
Funding
This work was supported by grants from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2015BAI12B04); Beijing Municipal Organization Department talents project (2015000021469G219); National Natural Science Foundation of China (81701137 and 81870904); and Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150501).
Author information
Authors and Affiliations
Contributions
Junsheng Li analyzed the results and wrote the manuscript. Peicong Ge made the statistical comparison. Qian Zhang and Fa Lin collected the patient data. Dong Zhang, Yan Zhang, and Rong Wang revised the manuscript. Jizong Zhao and Wen Wang designed the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee in Beijing Tiantan Hospital.
Informed consent
Patient informed consents were obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Ge, P., Zhang, Q. et al. Hyperhomocysteinemia is a risk factor for postoperative ischemia in adult patients with moyamoya disease. Neurosurg Rev 44, 2913–2921 (2021). https://doi.org/10.1007/s10143-021-01482-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01482-9