Abstract
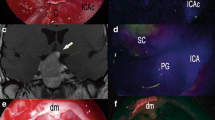
Microscopic indocyanine green videoangiography (mICG-VA) has gained wide acceptance during intracranial aneurysm surgery by lowering rates of incomplete clipping and occlusion of surrounding vessels. However, mICG-VA images are limited to the microscopic view and some deeper areas, including the aneurysm sac/neck posterior side, cannot be efficiently assessed as they are hidden by the aneurysm, clips, or surrounding structures. Contrarily, endoscopes allow a wider area of visualization, but neurosurgical endoscopes to date only provided visual data. We describe the first application of endoscope ICG-integrated technology (eICG) applied in an initial case of anterior communicating artery aneurysm clipping. This new technique provided also relevant information regarding aneurysm occlusion and patency of parent and branching vessels and small perforating arteries. eICG-VA provided additional information compared to mICG-VA by magnifying areas of interest and improving the ability to view less accessible regions, especially posterior to the aneurysm clip. Obtaining eICG sequences required currently the microscope to be moved away from the operating field. eICG-VA was only recorded under infrared illumination which prevented tissue handling, but white-infrared light views could be interchanged instantaneously. Further development of angled endoscopes integrating the ICG technology and dedicated filters blocking the microscopic light could improve visualization capacities even further. In conclusion, as a result of its ability to reveal structures around corners, the eICG-VA technology could be beneficial when used in combination with mICG-VA to visualize and confirm vessel patency in areas that were previously hidden from the microscope.




Similar content being viewed by others
References
Alexander TD, Macdonald RL, Weir B, Kowalczuk A (1996) Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery 39(1):10–17, discussion 17–18
Amin-Hanjani S, Meglio G, Gatto R, Bauer A, Charbel FT (2006) The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery 58(4 Suppl 2):ONS-305–ONS-312, discussion ONS-312
Bailes JE, Tantuwaya LS, Fukushima T, Schurman GW, Davis D (1997) Intraoperative microvascular Doppler sonography in aneurysm surgery. Neurosurgery 40(5):965–970, discussion 970–962
Betz CS, Zhorzel S, Schachenmayr H, Stepp H, Havel M, Siedek V, Leunig A, Matthias C, Hopper C, Harreus U (2009) Endoscopic measurements of free-flap perfusion in the head and neck region using red-excited indocyanine green: preliminary results. J Plast Reconstr Aesthet Surg 62(12):1602–1608
Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J (2009) Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol 71(5):543–550, discussion 550
David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S (1999) Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg 91(3):396–401
de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A (2007) Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 61(3 Suppl):63–72, discussion 72–63
Eloy JA, Carai A, Patel AB, Genden EM, Bederson JB (2008) Combined endoscope-assisted transclival clipping and endovascular stenting of a basilar trunk aneurysm: case report. Neurosurgery 62(3 Suppl 1):142–143, discussion 143–144
Ensenat J, Alobid I, de Notaris M, Sanchez M, Valero R, Prats-Galino A, Ferrer E (2011) Endoscopic endonasal clipping of a ruptured vertebral-posterior inferior cerebellar artery aneurysm: technical case report. Neurosurgery 69(1 Suppl Operative):onsE121–onsE127, discussion onsE127–128
Fischer G, Oertel J, Perneczky A (2011) Endoscopy in aneurysm surgery. Neurosurgery. doi:10.3205/11dgnc265
Fischer G, Stadie A, Oertel JM (2010) Near-infrared indocyanine green videoangiography versus microvascular Doppler sonography in aneurysm surgery. Acta Neurochir (Wien) 152(9):1519–1525
Germanwala AV, Zanation AM (2011) Endoscopic endonasal approach for clipping of ruptured and unruptured paraclinoid cerebral aneurysms: case report. Neurosurgery 68(1 Suppl Operative):234–239, discussion 240
Gruber A, Dorfer C, Standhardt H, Bavinzski G, Knosp E (2011) Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery 68(3):657–673, discussion 673
Hoh BL, Curry WT Jr, Carter BS, Ogilvy CS (2004) Computed tomographic demonstrated infarcts after surgical and endovascular treatment of aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 146(11):1177–1183
Ishihara R, Iishi H, Kidu T, Yamamoto S, Miyoshi R, Inoue T, Takeuchi Y, Higashino K, Uedo N, Tatsuta M (2008) Infrared endoscopic system for bleeding-point detection after flushing with indocyanine green solution (with videos). Gastrointest Endosc 68(5):975–981
Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P (2002) Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery 51(5):1119–1126, discussion 1126–1117
Kassam AB, Mintz AH, Gardner PA, Horowitz MB, Carrau RL, Snyderman CH (2006) The expanded endonasal approach for an endoscopic transnasal clipping and aneurysmorrhaphy of a large vertebral artery aneurysm: technical case report. Neurosurgery 59(1 Suppl 1):ONSE162–ONSE165, discussion ONSE162–165
Kinouchi H, Yanagisawa T, Suzuki A, Ohta T, Hirano Y, Sugawara T, Sasajima T, Mizoi K (2004) Simultaneous microscopic and endoscopic monitoring during surgery for internal carotid artery aneurysms. J Neurosurg 101(6):989–995
Kivisaari RP, Porras M, Ohman J, Siironen J, Ishii K, Hernesniemi J (2004) Routine cerebral angiography after surgery for saccular aneurysms: is it worth it? Neurosurgery 55(5):1015–1024
Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP, Zabramski JM, Porter RW, Albuquerque FC, McDougall CG, Fiorella DJ (2004) Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg 100(2):230–235
Leveque M, McLaughlin N, Laroche M, Bojanowski MW (2011) Endoscopic treatment of distal choroidal artery aneurysm. J Neurosurg 114(1):116–119
Litvack ZN, Zada G, Laws ER Jr (2012) Indocyanine green fluorescence endoscopy for visual differentiation of pituitary tumor from surrounding structures. J Neurosurg 116(5):935–941
Ma CY, Shi JX, Wang HD, Hang CH, Cheng HL, Wu W (2009) Intraoperative indocyanine green angiography in intracranial aneurysm surgery: microsurgical clipping and revascularization. Clin Neurol Neurosurg 111(10):840–846
Macdonald RL, Wallace MC, Kestle JR (1993) Role of angiography following aneurysm surgery. J Neurosurg 79(6):826–832
Perneczky A, Boecher-Schwarz HG (1998) Endoscope-assisted microsurgery for cerebral aneurysms. Neurol Med Chir (Tokyo) 38(Suppl):33–34
Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52(1):132–139, discussion 139
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103(6):982–989
Secer HI, Duz B, Solmaz I, Gonul E (2010) Endoscopic clipping of a middle cerebral artery aneurysm in a middle fossa arachnoid cyst and review of the literature. Minim Invasive Neurosurg 53(3):132–137
Sindou M, Acevedo JC, Turjman F (1998) Aneurysmal remnants after microsurgical clipping: classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir (Wien) 140(11):1153–1159
Tang G, Cawley CM, Dion JE, Barrow DL (2002) Intraoperative angiography during aneurysm surgery: a prospective evaluation of efficacy. J Neurosurg 96(6):993–999
Taniguchi M, Takimoto H, Yoshimine T, Shimada N, Miyao Y, Hirata M, Maruno M, Kato A, Kohmura E, Hayakawa T (1999) Application of a rigid endoscope to the microsurgical management of 54 cerebral aneurysms: results in 48 patients. J Neurosurg 91(2):231–237
Thines L, Dehdashti AR, Howard P, Da Costa L, Wallace MC, Willinsky RA, Tymianski M, Lejeune JP, Agid R (2010) Postoperative assessment of clipped aneurysms with 64-slice computerized tomography angiography. Neurosurgery 67(3):844–853, discussion 853–844
Turhan T, Ersahin Y (2011) Near-infrared camera for intraventricular neuroendoscopic procedures: in vitro comparison of the efficiency of near-infrared camera and visual light camera during bleeding. Childs Nerv Syst 27(3):439–444
Wang S, Liu L, Zhao Y, Zhang D, Yang M, Zhao J (2010) Evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. Neurosurg Rev 34(2):209–215
Acknowledgments
We thank Jeroen Coppens, MD, for his help and Justin Neira for his video narration.
Addendum
Since the submission of the manuscript on Feb 25th 2012 another article on the same subject has been published: Endoscopic indocyanine green video angiography in aneurysm surgery: an innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, Horikoshi T. J Neurosurg. 2012 Aug;117(2):302-8. Epub 2012 Jun 8.
Grant
Michaël Bruneau, MD, PhD, received a grant from the Fond National de la Recherche Scientifique (Belgium) for supporting the microscopic infrared module installation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Andreas Raabe, Bern, Switzerland
As often observed with progress in operative neurosurgery, the combination of two originally completely different methods conquers new territory. In this interesting report, the authors combine the use of an endoscope and indocyanine green (ICG) angiography, two modern technologies that were originally developed for selected procedures but have found their routine use in contemporary neurosurgery. While ICG angiography clearly has the limitation that contrary to digital substraction angiography only the visible structures can be assessed, the combined use with an endoscope may overcome this limitation. It may be of help in deep-seated surgical fields or when perforating arteries are hidden on the backside of the artery and run close to the clip branches. Yet, this still remains the future because the method described in this report used a 0° endoscope that provides a view similar as the surgical microscope. As discussed by the authors, the development of angled endoscopes with ICG technology will be the logical next step.
Giuseppe Esposito, Utrecht, The Netherlands Luca Regli, Utrecht, The Netherlands and Zurich, Switzerland
Microscopic indocyanine green videoangiography (mICG-VA) is known to be an excellent tool in neurosurgery by allowing a simple, reliable, fast, and noninvasive intraoperative observation and documentation of blood flow. mICG-VA image quality, spatial and temporal resolution, enables a real-time assessment of the cerebral circulation, with distinct evaluation of arterial, capillary, and venous phases (1, 2). One of the advantages of mICG-VA is the possibility to be repeated almost as many times as needed, within a maximum daily dose of 5 mg/kg, waiting about 5–10 min (for dye clearance) between two consecutive ICG administrations. The recommended dose for a single ICG administration is 0.2–0.5 mg/kg (2).
Since the first report in 2003 from Raabe et al. (1), mICG-VA became very popular in neurosurgery and many authors reported about its feasibility, safety, and efficacy in different neurosurgical procedures (1–10), essentially in the neurovascular area, where nowadays mICG-VA has become an invaluable instrument to intraoperative surgical decision-making.
In aneurysm surgery, mICG-VA is known to optimize the visualization of the angioanatomy, to assess the completeness of aneurysm occlusion as well as the patency of peri-aneurysmal vessels, including arteries with diameter of <0.5 mm (e.g., perforating arteries) (2, 3). In bypass surgery, mICG-VA permits to assess the patency of donor and recipient vessels (4); we also described its use for the correct identification of the recipient artery in selective cerebral revascularization surgery (5). In arteriovenous malformation surgery, mICG-VA has shown to be useful to clarify the lesion vascularization, to assess intraoperative flow variations and to confirm the occlusion of the arteries feeding the nidus (6). In dural arteriovenous fistulae surgery, mICG-VA can help in the identification and in the confirmation of surgical obliteration of the arteriovenous shunt (7). Further applications such as in extracranial vertebral artery surgery (8) or in tumor surgery (9) have been described.
Besides all the well-known advantages, major limitations of the mICG-VA exist. Either on the basis of the current literature or of our experience, one of the main limitations is that the observable territory is restricted to the microscopic view, so that some areas can remain hidden despite changing the orientation of the microscope or gentle manipulation of neurovascular structures. For instances, in aneurysms surgery, mICG-VA fails to show areas such as the posterior aneurysmal sac/neck, or vessels located behind the aneurysms or the clip itself. Moreover, ICG fluorescence signal is very poor in thick-walled or partially thrombosed/calcified aneurysms or in vessels covered by blood clots (2). Finally, the quality of mICG-VA images can be poor in deep located lesions. Neuroendoscopy is known to be able to improve visualization of areas that are hidden to the microscopic visualization, by increasing the number of angles of view and by magnification power (10).
In this article, Bruneau et al. reported the application of endoscopy-integrated ICG-VA technology (eICG-VA) in the treatment of a patient with an anterior communicating artery aneurysm, in combination with mICG-VA. The objectives of their work are to test the feasibility of eICG-VA and to evaluate the potential advantages and limitations.
The possibility to repeat multiple ICG administrations does not contraindicate the serial use of endoscopic and microscopic ICG-VA technology. The quality of the images provided by eICG-VA is very high, as confirmed by the visualization of small arteries such as perforators and recurrent Heubner. The spatial and temporal resolution allows a real-time evaluation of the angioanatomy and to obtain reliable information regarding aneurysm occlusion and post-clipping patency of the peri-aneurysmal vessels. eICG-VA can also be able to magnify the areas of work and to better visualize less accessible zones (e.g., behind the clip).
With the reported technology, to obtain eICG images, it is necessary to move the microscope away from the operative field. As stated by the authors, the introduction of new endoscopic filters could permit the contemporary recording of both endoscopic and microscopic modalities. Furthermore, the endoscopic device used by Bruneau et al. is bigger (5.8 mm diameter) when compared with the endoscopes currently used in neurosurgical practice. So with the current instrumentation, it could be difficult to reach lesions located in deep spaces, because a narrow surgical corridor could not permit the introduction of the endoscope. Bruneau et al. also worked with a 0° endoscope which definitely limited the visualization of peri-aneurysmal vascular anatomy.
It is important to mention the very recent results reported by Nishiyama et al. (11) who demonstrated the efficacy and the safety of the combined use of eICG-VA and mICG-VA in a case series of three patients with unruptured intracranial aneurysms. The intended and appropriate position of clip blade was confirmed by eICG-VA, as well as the patency of perforating arteries lying behind the aneurysm and the parent vessels (not visible under mICG-VA). The viewing angle of the endoscopes used by the authors was 30° or 70°, and the outer diameter was 4.0 mm. Moreover, the applied technology offered the possibility to simultaneously observe the fluorescence under the surgical microscope and via the endoscope.
We strongly encourage further developments of eICG-VA technology in order to (1) obtain different angled endoscopes (0°, −30°, −45°, −70°, and −110°) to provide more perspectives for the exploration of the lesions and peri-lesion angioanatomy and (2) to reduce the size of the current endoscopic device to permit its application in deeper areas as well as in minimally invasive neurosurgical strategies. This will certainly make eICG-VA technology a very important tool in neurovascular surgery.
References
1. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 2003; 52 (1): 132–139; discussion 139.
2. Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J (2009) Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol 71: 543–550.
3. de Oliveira M, Beck J, Seifert V, Teixeira MJ, Raabe A (2007) Assessment of blood in perforating arteries during intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 61 (3 Suppl): 63–72; discussion 72–73.
4. Woitzik J, Horn P, Vajkoczy P, Schmiedek P (2005) Intraoperative control of extracranial–intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102: 692–698.
5. Esposito G, Durand A, van Doormaal TP, Regli L (2012) Selective-targeted extra-intracranial bypass surgery in complex middle cerebral artery aneurysms: correctly identifying the recipient artery using indocyanine green video-angiography. Neurosurgery (in press).
6. Takagi Y, Sawamura K, Hashimoto N, Miyamoto S (2012) Evaluation of serial intraoperative surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography in patients with cerebral arteriovenous malformations. Neurosurgery 70(1 Suppl Operative):34–42; discussion: 42–43.
7. Schuette AJ, Cawley CM, Barrow DL (2010) Indocyanine green videoangiography in the management of dural arteriovenous fistulae. Neurosurgery 67(3):658–62; discussion 662.
8. Bruneau M, Sauvageau E, Nakaji P, Vandesteene A, Lubicz B, Chang SW, Balériaux D, Brotchi J, De Witte O, Spetzler RF (2010) Preliminary personal experiences with the application of near-infrared indocyanine green videoangiography in extracranial vertebral artery surgery. Neurosurgery 66(2):305–311; discussion 311.
9. Ferroli P, Acerbi F, Albanese E, Tringali G, Broggi M, Franzini A, Broggi G (2011) Application of intraoperative indocyanine green angiography for CNS tumors: results on the first 100 cases. ActaNeurochir Suppl 109:251–257.
10. Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P (2002) Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery 51 (5):1119–1126; discussion 1126–1127.
11. Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, Horikoshi T (2012) Endoscopic indocyanine green video angiography in aneurysm surgery: an innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg 117(2):302–308.
Electronic supplementary material
Below is the link to the electronic supplementary material.
This video shows the images from the microscopic and endoscopic indocyanine green videoangiographies performed during the surgical clipping of an unruptured anterior communicating artery aneurysm (M4V 24793 kb)
Rights and permissions
About this article
Cite this article
Bruneau, M., Appelboom, G., Rynkowski, M. et al. Endoscope-integrated ICG technology: first application during intracranial aneurysm surgery. Neurosurg Rev 36, 77–85 (2013). https://doi.org/10.1007/s10143-012-0419-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-012-0419-9