Abstract
The authors report on 84 patients with single melanoma brain metastasis surgically treated from 1997 to 2007. There were 46 males and 38 females; mean age was 41 years (range 24–58 years). All patients were surgically treated, and 52 of them received postoperative adjuvant therapy consisting of whole-brain radiation therapy (36), radiosurgery (9), or a combination of these two techniques (7). Brain recurrences were observed in 44 cases, of which 9 were local. Of the latter, seven were re-operated while the remaining two were treated by radiosurgery. At 1-year follow-up, the survival rate was 52% (32 patients) whereas only 12 patients (14%) were still alive after 2 years. None of the patients in which removal was subtotal survived for more than 6 months after surgical treatment. Three years after the onset of the brain metastasis, five patients (6%) were still alive. Survival was significantly influenced by treatment with regard to overall survival reported in other series. A review of literature, together with our own series, suggests that radical surgical treatment of the lesion possibly employing the internal no-touch technique has significantly increased survival in our patients (p < 0.05) and that the association of postoperative radiotherapy and re-operation in the event of recurrent metastatic lesions is advisable even though statistical significance was not reached (p > 0.05).



Similar content being viewed by others
References
Alexander E III, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, Kooy HM, Loeffler JS (1995) Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst 87:34–40
Atkins M, Sosman J, Agarwala S, Logan T, Clark JI, Ernstoff MS, Lawson D, Dutcher JP, Weiss G, Curti B, Margolin KA (2008) Temozolomide, thalidomide, and whole brain radiation therapy for patient with brain metastasis from metastatic melanoma. Cancer 113:2139–2145
Bindal AK, Bindal RK, Hess KR, Shiu A, Hassenbusch SJ, Shi WM, Sawaya R (1996) Surgery versus radiosurgery in the treatment of brain metastases. J Neurosurgery 84:748–754
Breslow A (1970) Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 172:902–908
Brown PD, Brown CA, Pollock BE, Gorman DA, Foote RL (2002) Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery 51:656–666
Buchsbaum JC, Suh JH, Lee SY, Chiedel MA, Greskovich JF, Barnett GH (2002) Survival by radiation therapy oncology group recursive partitioning analysis class and treatment modality in patients with brain metastasis from malignant melanoma: a retrospective study. Cancer 94:2265–2272
Clark WH Jr, From L, Bernardino EA et al (1969) The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 29:705–726
Douglas JG, Margolin K (2002) The treatment of brain metastases from malignant melanoma. Semin Oncol 29(5):518–524
Gaspar L, Scott C, Rotmann M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trial. Int J Radiat Oncol Biol Phys 37(4):745–751
Gieger M, Wu JK, Ling MN, Wazer D, Tsai JS, Engler MJ (1997) Response of intracranial melanoma metastases to stereotactic radiosurgery. Radiat Oncol Investig 5:72–80
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kleihues P, Cavanee WK (2000) World Health Classification of tumors: pathology and genetics of tumors of the nervous system. IARC Press, Lyon
Mingione V, Oliveira M, Prasad D, Steiner M, Steiner L (2002) Gamma surgery for melanoma metastases in the brain. J Neurosurg 96:544–551
Patel A, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick D, Lang FF, Sawaya R (2010) Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 113(2):181–189
Raizer J, Hwu W, Panageas KS, Wilton A, Baldwin DE, Bailey E, von Althann C, Lamb LA, Alvarado G, Bilsky MH, Gutin PH (2008) Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol 10:199–207
Redmond A, Di Luna ML, Hebert R, Moliterno JA, Desai R, Knisely JP, Chiang VL (2008) Gamma knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg 109:99–105
Salvati M, Cervoni L, Caruso L, Gagliardi M (1996) Solitary cerebral metastasis from melanoma: value of en bloc resection. Clin Neurol Neurosurg 98:12–14
Sampson JH, Carter JH, Friedman AH, Seigler HF (1998) Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurgery 88:11–20
Sawaya R, Ligon BL, Bindal AK, Bindal RK, Hess KR (1996) Surgical treatment of metastatic brain tumors. J Neurooncology 27:269–277
Sloan AE, Nock CJ, Einstein DB (2009) Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 16:248–255
Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, Sawaya R (2009) Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 64:644–676
Wronski M, Arbit E (2000) Surgical traeatment of brain metastases from melanoma: a retrospective study of 91 patients. J Neurosurgery 93:9–18
Zacest A, Besser M, Stevens G, Thompson JF, Mc Carthy W, Culjak G (2002) Surgical management of cerebral metastases from melanoma: outcome in 147 patients treated at a single institution over two decades. J Neurosurgery 96:552–558
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Silvia Hofer, Zürich, Switzerland
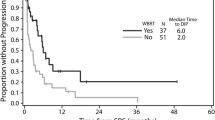
The authors report about a remarkable cohort of patients (n = 84) with single brain metastases from melanoma histology only. This retrospective series confirms that WBRT might be omitted as an adjuvant postoperative treatment since it does not affect overall survival. This has already been demonstrated in a large randomized trial by the European Organisation for Research and Treatment of Cancer (EORTC) in patients with one to three brain metastases of solid tumors [1]. Furthermore, the series demonstrates that patients with single brain metastases but with systemic disease do worse compared to solitary brain metastases (brain disease only) as we know from large RTOG trials for brain metastases of any histology using RPA, a prognostic index [2].
In line with results from other and one recently published retrospective cohort (n = 240), median survival in patients with single brain metastases from melanoma is around 7 months, which is slightly better than in patients with multiple brain metastases, most probably due to more effective local treatment (surgery and stereotactic radiosurgery) [3]. Neuroradiological follow-up for patients at high risk for CNS recurrence from melanoma, as suggested by the authors, seems not reasonable with currently available tools. The ESMO guidelines 2010 state that there is no consensus on the frequency and use of imaging techniques in the follow-up of a melanoma patient. Rising serum S-100 levels are the most accurate blood test in the follow-up of melanoma patients, if any blood test is recommended at all [4]. Although still in clinical trials, BRAF-Inhibitors and ipilimumab are worth mentioning as therapeutic options, even in the context of brain metastases in metastatic melanoma (www.clinicaltrials.gov).
References
1. Kocher M, Soffietti R, Abacioglu U et al. (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141
2. Gaspar L, Scott C, Rotman M et al. (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Oncol Biol Phys 37:745–751
3. Eigentler T, Figl A, Krex D et al. (2011) Number of metastases, serum lactate dehydrogenase level and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer 117(8):1697–1703
4. Dummer R, Hauschild A, Guggenheim M et al. (2010) Melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol 21(suppl 5):v194–197
Gabriele Schackert, Dresden, Germany
The publication by Salvati and colleagues covers the important issue of treatment of cerebral metastases. The authors focus on brain metastases of the malignant melanoma, which is known to be the primary tumor with the highest incidence for cerebral seeding.
Eighty-six patients were included in the study. All patients had only one single cerebral lesion, and all were treated by surgery. Adjuvant therapy comprised radiotherapy, radiosurgery, or a combination of these two modalities.
An important finding was that none of the patients, in which removal was subtotal, survived more than 6 months. Patients benefit from complete resection, employing the no-touch technique. Thirty-two patients were treated by surgery alone, 36 patients by adjuvant radiotherapy, 9 by adjuvant radiosurgery, and the remaining by a combination of both techniques.
At 1-year follow-up, the survival rate was 52% (32 patients); at 2 years, only 12 patients were still alive; at 3 years, 5 patients. In 52.3% (44 patients), death was related to neurological causes.
The paper has three messages:
1. Complete removal of a metastasis can lead to prolonged survival. The overall diameter of the lesion and the quantity of peritumoral edema were found to be the fundamental parameters for the outcome of the patients. Lesions bigger than 3 cm3 were considered as an absolute indication for surgical treatment. Smaller lesions were optional. The authors point out that the “en bloc” resection is crucial for the avoidance of a local recurrence. This has been also published by Patel and collegues recently in 2010 [1]. However, Salvati and colleagues do not discuss the presence of an infiltration zone around the lesion, which has also been shown previously [2]. The microscopic resection including a safety zone of half a centimeter around the lesion can lead to a significant better local tumor control [3]. If we want to improve our surgical results, both aspects—en bloc resection and infiltration zone—should be respected.
2. Radiotherapy as an adjunct did not improve the overall survival; however it reduced significantly the incidence of recurrence at local and distant sites. This already has been shown by several prospective randomized trials, covering metastases of different origin. Melanoma metastases are known to be low radiosensitive. Therefore, the decision against adjuvant postsurgical WBRT might be logical. We have the same regimen in the treatment of single cerebral metastasis in our institution. We rely on the postoperative MRI. If no residual tumor can be demonstrated, adjuvant radiotherapy will not be applied. In case of residual tumor, we go for WBRT or radiosurgery.
3. In case of recurrence, the authors favor active treatment, e.g., re-operation of local or distant metastases, which improves survival and quality of life. This again is our experience. The decision for active treatment of recurrent lesions: re-operation, radiosurgery, or radiotherapy, however, should mainly depend on prognostic factors, e.g., the KPS and the progress of the extracranial tumor burden.
References
1. Patel AJ et al. (2010) Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 113:181–189
2. Baumert BG et al. (2006) A pathology-based substrate for target definition of brain metastases. Int J Radiat Oncol Biol Phys 66:187–194
3. Yoo H et al. (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 110:730–736
Rights and permissions
About this article
Cite this article
Salvati, M., Frati, A., D’Elia, A. et al. Single brain metastases from melanoma: remarks on a series of 84 patients. Neurosurg Rev 35, 211–218 (2012). https://doi.org/10.1007/s10143-011-0348-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-011-0348-z