Abstract
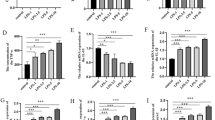
The objective of this study was to investigate the impact of formononetin on cellular apoptosis and inflammatory responses following spinal cord injury (SCI), as well as the underlying mechanisms involved. In this study, PC12 cells were treated with lipopolysaccharide (LPS) and different concentrations of Formononetin (FT) (50 μM, 100 μM, 200 μM). To confirm the effect of nuclear factor-κB (NF-κB)/NLR family pyrin domain containing 3 (NLRP3) signaling pathways, the cells in the phorbol-12-myristate-13-acetate (PMA) group were treated with 0.1 μmol/L PMA (NF-κB/NLRP3 signaling pathway activators). The lactate dehydrogenase (LDH) concentration and cell viability, proliferating cell nuclear antigen (PCNA) fluorescence intensity, and cell apoptosis were determined using an LDH kit, Cell Counting Kit-8 (CCK-8), immunofluorescence, and flow cytometry assays, respectively. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-16 (IL-6) expression levels were detected by quantitative ELISA assay. The expression of proteins related to the NF-κB/NLRP3 signaling pathway was detected by western blotting. Our results showed that LPS increased LDH levels in PC12 cells, suggesting that inflammation caused PC12 cell damage. However, the PC12 cell damage was decreased by methylprednisolone. Formononetin promotes cell survival and proliferation, and prevents apoptosis in a concentration-dependent manner. Formononetin reduced the TNF-α, IL-1β, and IL-6 levels in the LPS-treated model. Moreover, formononetin decreased the levels of p-p65 NF-κB and NLRP3 in PC12 cells. We conclude that formononetin ameliorated the inflammatory response and apoptosis in LPS-induced inflammatory injury in neuronal cells via the NF-κB/NLRP3 signaling pathway.




Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Abbaszadeh F, Fakhri S, Khan HJPR (2020) Targeting Apoptosis and Autophagy following Spinal Cord Injury: Therapeutic Approaches to Polyphenols and Candidate Phytochemicals. Pharmacol Res 160:105069
Adeeb N, Deep A, Hose N, Rezaei M, Fard SA, Tubbs RS, Yashar P, Liker MA, Kateb B, Mortazavi MM 2015 Stem cell therapy for spinal cord injury: The use of oligodendrocytes and motor neurons derived from human embryonic stem cells. J Translational Research in Anatomy 1:17–24.
Bastian C, Zerimech S, Nguyen H et al (2022) Aging astrocytes metabolically support aging axon function by proficiently regulating astrocyte-neuron lactate shuttle. Exp Neurol 357:114173. https://doi.org/10.1016/j.expneurol.2022.114173
Cai J, Ziemba KS, Smith GM, Jin YJJ (2010) Evaluation of cellular organization and axonal regeneration through linear PLA foam implants in acute and chronic spinal cord injury. J Neuroinflammation 83A:512–520
Chao W, Lu Z, Ndong JDLC et al (2019) Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J Neuroinflammation 16(1):238
Chen G, Lin C, Zhu Z et al (2023) Increased blood flow of spinal cord lesion after decompression improves neurological recovery of degenerative cervical myelopathy: an intraoperative ultrasonography-based prospective cohort study. Int J Surg 109:1149–1157. https://doi.org/10.1097/JS9.0000000000000361
Cho Y, Shi R, Ivanisevic A, Ben Borgens R (2010) Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J Neurosci Res 88:1433–1444
CG Meireles, CL de Lima, MM de Paula Oliveira et al (2021) Antiproliferative effects of metformin in cellular models of pheochromocytoma. Mol Cell Endocrinol 539:111484. https://doi.org/10.1016/j.mce.2021.111484
Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D (2019) Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci 20(11):2698. https://doi.org/10.3390/ijms20112698
Dolci S, Mannino L, Bottani E et al (2022) Therapeutic Induction of Energy Metabolism Reduces Neural Tissue Damage and Increases Microglia Activation in Severe Spinal Cord Injury. J Neuroinflam 178:106149
Fan H, Tang HB, Shan LQ, Liu SC, Huang DG, Chen X, Chen Z, Yang M, Yin XH, Yang H, Hao DJ (2019) Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammation 16(1):206. https://doi.org/10.1186/s12974-019-1613-2
Fang Y, Ye J, Zhao B, Sun J, Cao PJRB (2020) Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biology 36:101677
Gilbert EAB, Lakshman N, Lau KSK, Morshead CMJC (2022) Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair. Cells 11:846
Gu C, Li L, Huang Y et al (2020) Salidroside Ameliorates Mitochondria-Dependent Neuronal Apoptosis after Spinal Cord Ischemia-Reperfusion Injury Partially through Inhibiting Oxidative Stress and Promoting Mitophagy. Oxidative Med Cell Longev 2020:1
Guo S, Lin T, Chen G et al (2023) METTL3 Affects Spinal Cord Neuronal Apoptosis by Regulating Bcl-2 m6A Modifications After Spinal Cord Injury. Neurospine 20:623–636. https://doi.org/10.14245/ns.2346170.085
Herzog RI, Jiang L, Herman P, Zhao C (2013) Behar KLJTJoci Lactate Preserves Neuronal Metabolism and Function following Antecedent Recurrent Hypoglycemia. Neurospine 123(1988):1998
Hou Y, Luan J, Huang T et al (2021) Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J Neuroinflammation 18(1):216. https://doi.org/10.1186/s12974-021-02248-2
Huang Q, Duan W, Sivanesan E et al (2019) Spinal Cord Stimulation for Pain Treatment After Spinal Cord Injury. Neurosci Bull 35:539
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18:2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Jazayeri SB, Maroufi SF, Mohammadi E et al (2023) Incidence of traumatic spinal cord injury worldwide: A systematic review, data integration, and update. World Neurosurg X 18:100171. https://doi.org/10.1016/j.wnsx.2023.100171
Jin JH, Perez MA (2020) Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 5:1368
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20(13):3328. https://doi.org/10.3390/ijms20133328
Kippenberger AG, Palmer DJ, Comer AM, Lipski J, Burton LD, Christie DL (1999) Localization of the noradrenaline transporter in rat adrenal medulla and PC12 cells: evidence for its association with secretory granules in PC12 cells. J Neurochem 73(3):1024–1032
Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, Zhang X, Wang LL (2019) Mitochondrial transfer from bone marrow Mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics 9(7):2017–2035
Liu Z, Yao X, Jiang W et al (2020) Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J Neuroinflammation 17(1):90. https://doi.org/10.1186/s12974-020-01751-2
Liu H, Zhang J, Xu X, Lu S, Teng HJT (2021) SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics 11:4187–4206
Lukacova N, Kisucka A, Bimbova KK, Bacova M, Ileninova M, Kuruc T, Galik J (2021) Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int J Mol Sci 22(24):13577. https://doi.org/10.3390/ijms222413577
Masaki T, Yasuhisa A, Hisashi K, Noriyoshi S, Shunichi SJJNEN (2003) Ependymal cell reactions in spinal cord segments after compression injury in adult rat. J Neuroinflammation 17:185–194
Petrovic A, Veeraraghavan P, Olivieri D, Nistri A, Jurcic N, Mladinic M (2019) Loss of inhibitory synapses causes locomotor network dysfunction of the rat spinal cord during prolonged maintenance in vitro. Brain Res 1710:8–21
Pottorf TS, Rotterman TM, McCallum WM, Haley-Johnson ZA, Alvarez FJ (2022) The role of microglia in neuroinflammation of the spinal cord after peripheral nerve injury. Cells 11(13):2083. https://doi.org/10.3390/cells11132083
Rong Y, Liu W, Wang J, Fan J, Luo Y, Li L, Kong F, Chen J, Tang P, Cai W (2019) Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis 10(5):340. https://doi.org/10.1038/s41419-019-1571-8
Schmidt RL, Lenz LL (2012) Distinct licensing of IL-18 and IL-1β secretion in response to NLRP3 inflammasome activation. PLoS One 7(9):e45186. https://doi.org/10.1371/journal.pone.0045186
Su XQ, Wang XY, Gong FT, Feng M, Bai JJ, Zhang RR, Dang XQ (2020) Oral treatment with glycyrrhizin inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization after traumatic spinal cord injury. Brain Res Bull 158:1–8. https://doi.org/10.1016/j.brainresbull.2020.02.009
Tian J, Wang XQ, Tian Z (2022) Focusing on Formononetin: Recent Perspectives for its Neuroprotective Potentials. Front Pharmacol 13:905898. https://doi.org/10.3389/fphar.2022.905898
Tran AP, Warren PM, Silver J (2022) New insights into glial scar formation after spinal cord injury. Cell Tissue Res 387(3):319–336. https://doi.org/10.1007/s00441-021-03477-w
Wang DS, Yan LY, Yang DZ et al (2020) Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun 525:759
Wedel S, Mathoor P, Rauh O et al (2022) SAFit2 reduces neuroinflammation and ameliorates nerve injury-induced neuropathic pain. J Neuroinflammation 19:254
Wu QL, Cheng YQ, Liu AJ, Zhang WD (2020) Formononetin recovered injured nerve functions by enhancing synaptic plasticity in ischemic stroke rats. Biochem Biophys Res Commun S0006-291X(20)30281-3. https://doi.org/10.1016/j.bbrc.2020.02.015
Wu YQ, Xiong J, He ZL, Yuan Y, Wang BN, Xu JY, Wu M, Zhang SS, Cai SF, Zhao JX, Xu K, Zhang HY, Xiao J (2021) Metformin promotes microglial cells to facilitate myelin debris clearance and accelerate nerve repairment after spinal cord injury. Acta Pharmacol Sin 43(6):1360–1371
Yanagisawa S, Katoh H, Imai T, Nomura S, Watanabe M (2019) The relationship between inflammasomes and the endoplasmic reticulum stress response in the injured spinal cord. Neurosci Lett 705:54–59. https://doi.org/10.1016/j.neulet.2019.04.033
Yao X, Sun C, Fan B et al (2021) Neurotropin exerts neuroprotective effects after spinal cord injury by inhibiting apoptosis and modulating cytokines. J Orthop Translat 26:74–83
Yi L, Lu Y, Yu S, Cheng Q, Yi L (2022) Formononetin inhibits inflammation and promotes gastric mucosal angiogenesis in gastric ulcer rats through regulating NF-κB signaling pathway. J Recept Signal Transduct Res 42(1):16–22
Yu L, Zhang Y, Chen Q et al (2022) Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed Pharmacother 149:112836
Zendedel A, Johann S, Mehrabi S, Joghataei MT, Hassanzadeh G, Kipp M, Beyer CJMN (2016) Activation and Regulation of NLRP3 Inflammasome by Intrathecal Application of SDF-1a in a Spinal Cord Injury Model. Mol Neurobiol 53:3063–3075
Zhengzhao L, Guang Z, Xiaowen Z et al (2018) Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed Pharmacother 106:349–354
Zhou Y, Li L, Mao C, Zhou D (2022) Astragaloside IV ameliorates spinal cord injury through controlling ferroptosis in H(2)O(2)-damaged PC12 cells in vitro. Ann Transl Med 10:1176. https://doi.org/10.21037/atm-22-5196
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
PZ conceived and designed the study. ZZ experimented and drafted the manuscript. ZZ and PZ performed the literature search and data analysis. ZZ and PZ revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent participate
The experimental protocols were approved by the Ethics Committee of the Lianyungang Hospital of Traditional Chinese Medicine. No patient was involved in this study.
Animal ethics
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Zhang, P. Formononetin ameliorates the LPS-induced inflammatory response and apoptosis of neuronal cells via NF-κB/NLRP3 signaling pathway. Funct Integr Genomics 23, 321 (2023). https://doi.org/10.1007/s10142-023-01247-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01247-1