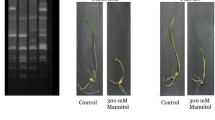
Abstract
Understanding the genes that govern tea plant (Camellia sinensis) architecture and response to drought stress is urgently needed to enhance breeding in tea with improved water use efficiency. Field drought is a slow mechanism and the plants go through an adaptive process in contrast to the drastic changes of rapid dehydration in case of controlled experiments. We identified a set of drought responsive genes under controlled condition using SSH, and validated the identified genes and their pattern of expression under field drought condition. The study was at three stages of water deficit stress viz., before wilting, wilting and recovery, which revealed a set of genes with higher expression at before wilting stage including dehydrin, abscissic acid ripening protein, glutathione peroxidase, cinnamoyl CoA reductase, calmodulin binding protein. The higher expression of these genes was related with increase tolerance character of DT/TS-463 before wilting, these five tolerant progenies could withstand drought stress and thus are candidates for breeding. We observed that physiological parameter like water use efficiency formed a close group with genes such as calmodulin related, DRM3, hexose transporter, hydrogen peroxide induced protein, ACC oxidase, lipase, ethylene responsive transcription factor and diaminopimelate decarboxylase, during wilting point. Our data provides valuable information for the gene components and the dynamics of gene expression in second and third leaf against drought stress in tea, which could be regarded as candidate targets potentially associated with drought tolerance. We propose that the identified five tolerant progenies on the basis of their drought tolerance can thus be utilised for future breeding programmes.














Similar content being viewed by others
References
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosakawa D, Shinozaki K (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9:859–1868
Annual Scientific Report (2006–07) Tocklai Experimental Station. Tea Research Association, Jorhat, India, pp 25–26
Bartels D, Singh M, Salamini F (1988) Onset of desiccation tolerance during development of the barley embryo. Planta 175:485–492
Bharalee R, Gupta S, Bhorali P, Bandyopadhyay T, Gohain B, Das SK, Saikia J, Borchetia S, Kalita MC, Handique AK, Das Sudripta (2011) Analysis of tea transcriptome for identification of genes related to drought tolerance. Genomics (in press)
Bouche N, Scharlat A, Snedden W, Bouchez D, Fromm H (2002) A novel family of calmodulin-binding transcription activators in multicellular organisms. J Biol Chem 277:21851–21861
Bray E (2002) Classification of genes differentially expressed during water deficit stress in Arabidopsis thaliana: an analysis using microarray and differential expression data. Ann Bot 89:803–811
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry & Molecular Biology of Plants. MD American Society of Plant Physiologists, Rockville, pp 1158–1249
Campbell SA, Close TJ (1997) Dehydrins: genes, proteins, and associations with phenotypic traits. New Phytol 137:61–74
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30:239–264
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97:795–803
Coraggio I, Tuberosa R (2004) Improving crops tolerance to abiotic stresses. In: Christou P, Klee H (eds) Handbook of Plant Biotechnology. John Wiley & Sons, Ltd, Chichester, pp 413–468
DaMatta FM, Chaves ARM, Pinheiro HA, Ducati C, Loureiro ME (2003) Drought tolerance of two field-grown clones of Coffea canephora. Plant Sci 164:111–117
David W (2002) Limitation to photosynthesis in water stressed leaves: stomata vs. metabolism and the role of ATP. Ann Bot 89:871–885
Dudley SA (1996) Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution 50:92–102
Dure L, Greenway S, Galau GA (1981) Developmental biochemistry of cotton seed embryogenesis and germination XIV. Changing mRNA populations as shown in vitro and in vivo protein synthesis. Biochemistry 20:4162–4168
El-Maarouf H, Arcy-Lameta A, Gareil M, Zuily-Fodil Y, Pham-Thi AT (2001) Cloning and expression under drought of cDNAs coding for two PI-PLCs in cowpea leaves. Plant Physiol Biochem 39:167–172
Frandsen G, Muller-Uri F, Nielsen M, Mundy J, Skriver K (1996) Novel plant Ca2+−binding protein expressed in response to abscisic acid and osmotic stress. J Biol Chem 271:343–348
Galau GA, Hughes DW, Dure L (1986) Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol 7:155–170
Gohain B, Borchetia S, Bhuyan LP, Rahman A, Sakata K, Mizutani M, Shimizu B, Gurusubramaniam G, Ravindranath R, Hazarika M, Das S (2012) Understanding Darjeeling Tea Flavour on a Molecular Basis. Plant Mol Biol 78:577–597. doi:10.1007/s11103-012-9887-0
Gonzalez and Gonzalez-Vilar (2001) Determination of relative water content. In: Handbook of plant ecophysiology techniques. MI Reigosa Roger (ed) Kluwer Publishers, Netherlands, pp 207–212
Hanson AS, Hitz WD (1982) Metabolic responses of plant water deficit. Ann Rev Plant Physiol 33:163–203
Heath LS, Ramakrishnan N, Sederoff RR, Whetten RW, Chevone BI, Struble CI, Jouenne VY, Chen D, van Zyl L, Grene R (2002) Studying the functional genomics of stress responses in loblolly pine with the Expresso microarray experiment management system. Comp Func Gen 3:226–243
Heschel MS, Donohue K, Hausmann NJ, Schmitt J (2002) Population differentiation and natural selection for water-use efficiency in Impatiens capensis (Balsaminaceae). Int J Plant Sci 163:907–912
Hirayama T, Ohto C, Mizoguchi T, Shinozaki K (1995) A gene encoding a phosphotidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 92:3903–3907
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Iturriga G, Leyns L, Villegas A, Gharabeih R, Salamini F, Bartels D (1996) A family of novel myb-related genes from the resurrection plant Craterostigma plantagineum is specifically expressed in callus and roots in response to ABA or desiccation. Plant Mol Biol 25:707–716
Iuchi S, Yamaguchi-Shinozaki K, Urao T, Terao T, Shinosaki K (1996) Novel drought inducible genes in the highly drought-tolerant cowpea: cloning of cDNAs and analysis of the expression of the corresponding genes. Plant Cell Physiol 37:1073–1082
Jang HJ, Pih KT, Kang SG, Lim JH, Jin JB, Piao HL, Hwang I (1998) Molecular cloning of a novel Ca2+ binding protein that is induced by NaCl stress. Plant Mol Biol 37:839–847
Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J 14:121–128
Kjellbom P, Larsson C, Johansson I, Karlsson M, Johanson U (1999) Aquaporins and water homeostasis in plants. Trends Plant Sci 25:308–314
Kong Z, Li M, Yang W, Xu W, Xue Y (2006) A novel nuclear localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol 141:1376–1388
Kotchoni SO, Bartels D (2003) Water stress induces the up-regulation of a specific set of genes in plants: aldehyde dehydrogenase as an example. Bulgarian J Plant Physiol (Special Issue): 37–51
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin-B like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 14:S389–S400
McKeon TA, Hoffmann NE, Yang SF (1982) The effect of plant-hormone pretreatments on ethylene production and synthesis of 1-aminocyclopropane-1-carboxylic acid in water-stressed wheat leaves. Planta 155:437–443
Mekliche A, Bouthier A, Gate P (1992) Analyse comparative des comportements à la sécheresse du blé dur et du blé tendre. In: Tolérance à la Sécheresse des Céréales en Zone Méditerranéenne, Diversité Génétique et Amélioration Variétale, Montpellier (France). INRA, Paris, Les Colloques, No 64, pp 15–17
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 93:765–769
Munnik T, Meijer HJG (2001) Osmotic stress activates distinct lipid and MAPK signalling pathways in plants. FEBS Lett 498:172–178
Ober ES, Sharp RE (1994) Proline accumulation in maize (Zea mays L.) primary roots at low water potentials: requirement for increased levels of abscisic acid. Plant Physiol 105:981–987
Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, Knight MR (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427:858–861
Sarda X, Tousch D, Ferrare K, Cellier F, Alcon C, Dupuis JM, Casse F, Lamaze T (1999) Characterization of closely related delta-TIP genes encoding aquaporins which are differentially expressed in sunflower roots upon water deprivation through exposure to air. Plant Mol Biol 25:179–191
Schaffner AR (1998) Aquaporin function, structure, and expression: Are there more surprises to surface in water relations? Planta 25:131–139
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 14:869–876
Singh ID, Handique AC (1993) Breeding for resistance in water stress in Tea. Two and a Bud 40(1):41-49
Snedden WA, Fromm H (1998) Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends Plant Sci 3:299–304
Snedden WA, Fromm H (2001) Calmodulin as a versatile calcium signal transducer in plants. New Phytol 15:35–66
Suprunova T, Krugman T, Fahima T, Chen G, Shams I, Korol A, Nevo E (2004) Differential expression of dehydrin genes in wild barley, Hordeum spontaneum, associated with resistance to water deficit. Plant Cell Environ 27:1297–1308
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important role of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29:417–426
Takahashi S, Katagiri T, Hirayama T, Yamaguchi-Shinozaki K, Shinozaki K (2001) Hyperosmotic stress induces a rapid and transient increase in Inositol1, 4, 5-triphosphate independent of abscisic acid in Arabidopsis cell cultures. Plant Cell Physiol 42:214–222
Tuteja N (2007) Mechanisms of high salinity tolerance in plants. Methods in Enzymology: Osmosensing Osmosignaling 428:419–438
Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194
Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737
Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5:1529–1539
Winicov I, Bastola DR (1999) Transgenic overexpression of the tran- scription factor Alfin1 enhances expression of the endogenous MSPRP2 gene in alfalfa and improves salinity tolerance of the plants. Plant Physiol 120:473–480
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Xiong L, Schumaker KS, Zhu JK (2002) Cell Signaling during Cold, Drought and Salt Stress. Plant Cell (Supplement): S165-S183
Yamada S, Komori T, Myers PN, Kuwata S, Kubo T, Imaseki H (1997) Expression of plama membrane water channel genes under water stress in Nicotiana excelsior. Plant Cell Physiol 25:1226–1231
Yang T, Poovaiah BW (2003) Calcium/Calmodulin-mediated signal network in plants. Trends Plant Sci 8:505–512
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124:941–948
Zhu JK (2002) Salt and drought stress signal transduction in plants. Ann Rev of Plant Biol 53:247–273
Acknowledgements
The authors thank Dr. M. Hazarika, Director TRA, Dr. Renu Swarup, Advisor, DBT, Govt. of India, and Dr. Anamika Gambhir, Principal Scientist, DBT for constant encouragement and support. The work was supported by generous funding from DBT, Govt. of India.
Accession numbers
EST data was deposited in the NCBI database (HS395562- HS396831).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sushmita Gupta and Raju Bharalee contributed equally.
Electronic supplementary material
The following materials are available in the online version of this article.
Supplementary Figure F1
Gene expression analysis of TV23 and S.3A/3 clones at three stages of induced drought for selection of transcripts. (DOC 93 kb)
Supplementary Table S1
Soil characteristics and weather condition of field drought experimental site. (DOC 32 kb)
Supplementary Table S2
The set up for the ligation reactions. (DOC 30 kb)
Supplementary Table S3
The set up for the first hybridization reaction. (DOC 30 kb)
Supplementary Table S4
GenBank accession number and primer pair sequences for the house keeping genes used as internal standards for Quantitative Real Time PCR analysis. (DOC 30 kb)
Supplementary Table S5
Homologies of drought induced unigenes of tea with the sequences in the NCBI database. (DOC 369 kb)
Supplementary Table S6
KEGG based pathway analysis of drought related unigenes of tea. The presence of enzyme is denoted by red colour and absence by green colour. (BWF = Before wilting forward library; BWR = Before wilting reverse library; WF = Wilting forward library; WR = Wilting reverse library). (DOC 98 kb)
Supplementary Table S7
Comparison of GOs based on biological process of drought responsive transcripts at two stages of drought stress. The presence of GOs is represented by red colour and absence by green colour. (BWF = Before wilting forward library; BWR = Before wilting reverse library; WF = Wilting forward library; WR = Wilting reverse library). (DOC 160 kb)
Supplementary Table S8
Comparison of GOs based on molecular function of drought responsive transcripts at two stages of drought stress. The presence of GOs is represented by red colour and absence by green colour. (BWF = Before wilting forward library; BWR = Before wilting reverse library; WF = Wilting forward library; WR = Wilting reverse library). (DOC 129 kb)
Supplementary Table S9
Comparison of GOs based on cellular component of drought responsive transcripts at two stages of drought stress. The presence of GOs is represented by red colour and absence by green colour. (BWF = Before wilting forward library; BWR = Before wilting reverse library; WF = Wilting forward library; WR = Wilting reverse library). (DOC 66 kb)
Rights and permissions
About this article
Cite this article
Gupta, S., Bharalee, R., Bhorali, P. et al. Identification of drought tolerant progenies in tea by gene expression analysis. Funct Integr Genomics 12, 543–563 (2012). https://doi.org/10.1007/s10142-012-0277-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-012-0277-0