Abstract
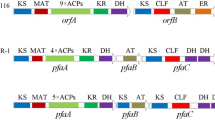
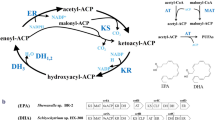
Schizochytrium sp. is a kind of marine microalgae with great potential as promising sustainable source of polyunsaturated fatty acids (PUFAs). Polyketide synthase-like (PKS synthase) is supposed to be one of the main ways to synthesize PUFAs in Schizochytrium sp. In order to study the exact relationship between PKS and PUFA biosynthesis, chain length factor (CLF) and dehydrogenase (DH) were cloned from the PKS gene cluster in Schizochytrium sp., then disrupted by homologous recombination. The results showed that DH- and CLF-disrupted strains had significant decreases (65.85 and 84.24%) in PUFA yield, while the saturated fatty acid (SFA) proportion in lipids was slightly increased. Meanwhile, the disruption of CLF decreased the C-22 PUFA proportion by 57.51% without effect on C-20 PUFA accumulation while DH-disrupted mutant decreased the production of each PUFA. Combined with analysis of protein prediction, it indicated that CLF gene exerted an enormous function on the carbon chain elongation in PUFA synthesis, especially for the final elongation from C-20 to C-22 PUFAs. Metabolomics analysis also suggested that the disruption of both genes resulted in the decrease of PUFAs but increase of SFAs, thus weakening glycolysis and tricarboxylic acid (TCA) cycle pathways. This study offers a broad new vision to research the mechanism of PUFA synthesis in Schizochytrium sp.







Similar content being viewed by others
References
Adkins Y, Kelley DS (2010) Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 21(9):781–792
Alonso DL, Maroto FG (2000) Plants as “chemical factories” for the production of polyunsaturated fatty acids. Biotechnol Adv 18(6):481–497
Bisang C, Long PF, Corté SJ, Westcott J, Crosby J, Matharu AL (1999) A chain initiation factor common to both modular and aromatic polyketide synthases. Nature 401(6752):502–505
Chang LKJ, Dumsday G, Nichols PD, Dunstan GA, Blackburn SI, Koutoulis A (2013a) High cell density cultivation of a novel Aurantiochytrium sp. strain TC 20 in a fed-batch system using glycerol to produce feedstock for biodiesel and omega-3 oils. Appl Microbiol Biotechnol 97(15):6907–6918
Chang G, Luo Z, Gu S, Wu Q, Chang M, Wang X (2013b) Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour Technol 142(8):255–260
Chang LKJ, Nichols CM, Blackburn SI, Dunstan GA, Koutoulis A, Nichols PD (2014) Comparison of Thraustochytrids aurantiochytrium sp., schizochytrium sp., thraustochytrium sp. and ulkenia sp. for production of biodiesel, long-chain omega-3 oils, and exopolysaccharide. Mar Biotechnol 16(4):396–411
Heath RJ, Rock CO (1996) Roles of the FabA and FabZ beta-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J Biol Chem 271(44):27795–27801
Hong WK, Heo SY, Oh BR, Kim CH, Sohn JH, Yang JW, Kondo A, Seo JW (2013) A transgene expression system for the marine microalgae Aurantiochytrium sp. KRS101 using a mutant allele of the gene encoding ribosomal protein L44 as a selectable transformation marker for cycloheximide resistance. Bioprocess Biosyst Eng 36(9):1191–1197
Huang J, Jiang X, Zhang X, Chen W, Tian B, Shu Z (2008) Expressed sequence tag analysis of marine fungus Schizochytrium producing docosahexaenoic acid. J Biotechnol 138:9–16
Kaulmann U, Hertweck C (2002) Biosynthesis of polyunsaturated fatty acids by polyketide synthases. Angew Chem Int Ed Engl 41(11):1866–1869
Keatingeclay AT, Maltby DA, Medzihradszky KF, Khosla C, Stroud RM (2004) An antibiotic factory caught in action. Nat Struct Mol Biol 11(9):888–893
Kueiling Y, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105(2):120–127
Li J, Ren LJ, Sun GN, Qu L, Huang H (2013) Comparative metabolomics analysis of docosahexaenoic acid fermentation processes by Schizochytrium sp. under different oxygen availability conditions. OMICS 17(5):269–281
Ling XP, Guo J, Liu X, Zhang X, Wang N, Lu Y, Ng IS (2014) Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. Lu310. Bioresour Technol 184:139–147
Lippmeier JC, Crawford KS, Owen CB, Rivas AA, Metz JG, Apt KE (2009) Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. Lipids 44(7):621–630
Liu B, Liu J, Sun P, Ma X, Jiang Y, Chen F (2015) Sesamol enhances cell growth and the biosynthesis and accumulation of docosahexaenoic acid in the microalga Crypthecodinium cohnii. J Agric Food Chem 63(23):5640–5645
Los DA, Mironov KS, Allakhverdiev SI (2013) Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth Res 116(2):489–509
Matsuda T, Sakaguchi K, Hamaguchi R, Kobayashi T, Abe E, Hama Y, Hayashi M, Honda D, Okita Y, Sugimoto S (2012) Analysis of Δ12-fatty acid desaturase function revealed that two distinct pathways are active for the synthesis of PUFAs in T. aureum ATCC 34304. J Lipid Res 53(6):1210–1222
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293(5528):290–293
Miao L, Wang Y, Chi S, Yan J, Guan G, Hui B, Li Y (2010) Reduction of fatty acid flux results in enhancement of astaxanthin synthesis in a mutant strain of Phaffia rhodozyma. J Ind Microbiol Biotechnol 37(6):595–602
Okuyama H, Orikasa Y, Nishida T, Watanabe K, Morita N (2007) Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl Environ Microbiol 73(3):665–670
Raghukumar S (2008) Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol 10(6):631–640
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815
Ren LJ, Huang H, Xiao AH, Lian M, Jin LJ, Ji XJ (2009) Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308. Bioprocess Biosyst Eng 32(6):837–843
Rogers LK, Valentine CJ, Keim SA (2013) DHA supplementation: current implications in pregnancy and childhood. Pharmacol Res 70(1):13–19
Sakaguchi K, Matsuda T, Kobayashi T, Ohara J, Hamaguchi R, Abe E, Nagano N, Hayashi M, Ueda M, Honda D (2012) Versatile transformation system that is applicable to both multiple transgene expression and gene targeting for Thraustochytrids. Appl Environ Microbiol 78(9):3193–3202
Song X, Tan Y, Liu Y, Zhang J, Liu G, Feng Y, Cui Q (2013) Different impacts of short-chain fatty acids on saturated and polyunsaturated fatty acid biosynthesis in Aurantiochytrium sp. SD116. J Agric Food Chem 61(41):9876–9881
Tang Y, Tsai SC, Khosla C (2003) Polyketide chain length control by chain length factor. J Am Chem Soc 125(42):12708–12709
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001) Essential fatty acids in visual and brain development. Lipids 36(9):885–895
Villasbôas SG, Bruheim P (2007) Cold glycerol-saline: the promising quenching solution for accurate intracellular metabolite analysis of microbial cells. Anal Biochem 370(1):87–97
Wallis JG, Watts JL, Browse J (2002) Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci 27(9):467–473
Warude D, Joshi K, Harsulkar A (2006) Polyunsaturated fatty acids: biotechnology. Crit Rev Biotechnol 26(2):83–93
Zhang AH, Xiao-Jun J, Wen-Jia W (2015) Lipid fraction and intracellular metabolite analysis reveal the mechanism of arachidonic acid-rich oil accumulation in the aging process of Mortierella alpine [J]. J Agric Food Chem 63(44):9812–9819
Funding
This work was financially supported by the Xiamen Southern Oceanographic Center (15GYY024NF03), the Natural Science Foundation of Fujian Province of China (No. 2017J01077), the University of Science and Technology in Fujian Province in the cooperative major project (2015H6004), and National Natural Science Foundation of China (51378444, 21676221). We also gratefully acknowledge Fujian Provincial Scientific and Technological Innovation Platform (2014H2006) for their continuous technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 1807 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Chen, X., Li, J. et al. Functions of PKS Genes in Lipid Synthesis of Schizochytrium sp. by Gene Disruption and Metabolomics Analysis. Mar Biotechnol 20, 792–802 (2018). https://doi.org/10.1007/s10126-018-9849-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9849-x