Abstract
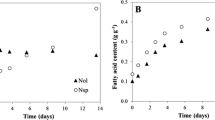
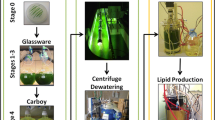
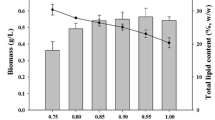
The microalgae Chlorella protothecoides UTEX 25, Chlorella sp. TISTR 8991, and Chlorella sp. TISTR 8990 were compared for use in the production of biomass and lipids under photoautotrophic conditions. Chlorella sp. TISTR 8990 was shown to be potentially suitable for lipid production at 30°C in a culture medium that contained only inorganic salts. For Chlorella sp. TISTR 8990 in optimal conditions in a stirred tank photobioreactor, the lipid productivity was 2.3 mg L−1 h−1 and after 14 days the biomass contained more than 30% lipids by dry weight. To attain this, the nitrogen was provided as KNO3 at an initial concentration of 2.05 g L−1 and chelated ferric iron was added at a concentration of 1.2 × 10−5 mol L−1 on the ninth day. Under the same conditions in culture tubes (36 mm outer diameter), the biomass productivity was 2.8-fold greater than in the photobioreactor (0.125 m in diameter), but the lipid productivity was only 1.2-fold higher. Thus, the average low-light level in the photobioreactor actually increased the biomass specific lipid production compared to the culture tubes. A light-limited growth model closely agreed with the experimental profiles of biomass production, nitrogen consumption, and lipid production in the photobioreactor.






Similar content being viewed by others
Abbreviations
- C N :
-
Concentration of potassium nitrate (g L−1)
- C N0 :
-
Initial concentration of potassium nitrate (g L−1)
- C P :
-
Concentration of lipid (g L−1)
- C X :
-
Concentration of biomass (g L−1)
- C X0 :
-
Initial concentration of biomass (g L−1)
- DOFFactor :
-
Degree of freedom of factors
- \( {\overline F_i} \) :
-
Averages of signal-to-noise ratio of factors at each factor level
- F ratio :
-
F-ratio
- I 0 :
-
Incident light level at the surface of the photobioreactor (μmol m−2 s−1)
- I r :
-
Local irradiance at radial distance r in the photobioreactor (μmol m−2 s−1)
- K a :
-
Light absorption coefficient of the algal biomass (cm2 g−1)
- K i :
-
Constant in Eq. 4 (μmol m−2 s−1)
- K s :
-
Saturation constant for light (μmol m−2 s−1)
- m N :
-
Nitrogen consumption constant for maintenance metabolism (g KNO3 g−1 biomass day−1)
- n :
-
Number of experiments
- q N :
-
Biomass specific uptake rate of potassium nitrate (g g−1 day−1)
- q P :
-
Biomass specific rate of lipid production (g g−1 day−1)
- Q P :
-
Volumetric production rate of lipids (g L−1 day−1)
- Q X :
-
Volumetric production rate of biomass (mg L−1 h−1)
- r :
-
Radial distance (m)
- R :
-
Radius of the photobioreactor (m)
- SSError :
-
Sum of squares of error
- SSFactor :
-
Sum of squares of factors
- S/N:
-
Signal-to-noise ratio
- t :
-
Time (days)
- \( \overline T \) :
-
Grand average of signal-to-noise ratio
- V Factor :
-
Variance of a factor
- y i :
-
The observed values of biomass or lipid concentrations (g L−1)
- Y opt :
-
The expected values of biomass or lipid concentrations (g L−1)
- Y P/X :
-
Lipid yield based on microalgal biomass (g lipid g−1 biomass)
- Y X/N :
-
Biomass yield based on potassium nitrate (g biomass g−1 KNO3)
- α :
-
Growth-associated lipid production constant (g lipid g−1 biomass)
- β :
-
Non-growth-associated lipid production constant (g lipid g−1 biomass day−1)
- μ r :
-
Local specific growth rate at radial position r in the photobioreactor (day−1)
- μ X :
-
Average specific growth rate in the photobioreactor (day−1)
- μ m :
-
Maximum specific growth rate (day−1)
References
Aiba S (1982) Growth kinetics of photosynthetic microorganisms. Adv Biochem Eng 23:85–156
ASTM (1976) Annual Book of ASTM Standards, Part 31, “Water”, Standard D 992-71, 363.
Banerjee A, Sharma R, Chisti Y, Banerjee UC (2002) Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Crit Rev Biotechnol 22:245–279
Camacho Rubio F, García Camacho F, Fernández Sevilla JM, Chisti Y, Molina Grima E (2003) A mechanistic model of photosynthesis in microalgae. Biotechnol Bioeng 81:459–473
Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E (2007) Metabolites from algae with economical impact. Comp Biochem Physiol C 146:60–78
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol 14:421–426
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chisti Y (2010a) Fuels from microalgae. Biofuels 1:233–235
Chisti Y (2010b) Shear sensitivity. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology, bioprocess, bioseparation and cell technology, vol 7. Wiley, New York, pp 4360–4398
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
García Camacho F, Molina Grima E, Sánchez Mirón A, González Pascual V, Chisti Y (2001) Carboxymethyl cellulose protects algal cells against hydrodynamic stress. Enzyme Microb Technol 29:602–610
García Camacho F, Gallardo Rodríguez J, Sánchez Mirón A, Cerón García MC, Belarbi EH, Chisti Y, Molina Grima E (2007) Biotechnological significance of toxic marine dinoflagellates. Biotechnol Adv 25:176–194
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Horikoshi T, Nakajima A, Sakaguchi T (1981) Accumulation of uranium by Chlorella cells grown under autotrophic, heterotrophic and mixotrophic culture conditions. Agric Biol Chem 45(3):781–783
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
Işik O, Sarihana E, Kuşvuran E, Gül Ö, Erbatur O (1999) Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minitum and Chlorella vulgaris in the algae–rotifer–fish larvae food chains. Aquaculture 174:299–311
Li Y, Horsman M, Wang B, Wu N, Christopher QL (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Liu Z-T, Weng G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Mazzuca Sobczuk T, Chisti Y (2010) Potential fuel oils from the microalga Choricystis minor. J Chem Technol Biotechnol 85:100–108
Mazzuca Sobczuk T, García Camacho F, Molina Grima E, Chisti Y (2006) Effects of agitation on the microalgae Phaeodactylum tricornutum and Porphyridium cruentum. Bioprocess Biosyst Eng 28:243–250
Molina Grima E, Acién Fernández FG, García Camacho F, Chisti Y (1999) Photobioreactors: light regime, mass transfer, and scaleup. J Biotechnol 70:231–247
Molina E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Rao RS, Prakasham RS, Prasad KK, Rajesham S, Sarma PN, Rao LV (2004) Xylitol production by Candida sp.: parameter optimization using Taguchi approach. Process Biochem 39:951–956
Richmond A (2004) Biological principles of mass cultivation. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 125–177
Roy RK (1990) A primer on the Taguchi method. VNR, New York, p 247
Roy RK (2001) Design of experiments using the Taguchi approach. Wiley, Toronto, p 538
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Curr Opin Biotechnol 21. doi:10.1016/j.copbio.2010.03.005.
Scragg AH, Illman AM, Carden A, Shales SW (2002) Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 23:67–73
Shuler ML, Kargi F (2002) Bioprocess engineering. Prentice-Hall, New Jersey, p 553
Singhasuwan S, Choorit W, Phoopat N, Parakulsuksatid P, Vanichsriratana W, Sirisansaneeyakul S (2009) Screening microalgae isolated in Thailand for the production of biodiesel. The Proceedings of 47th Kasetsart University Annual Conference, vol 8, pp. 386–391, Kasetsart University, Bangkok, Thailand.
Sirisansaneeyakul S, Luangpipat T, Vanichsriratana W, Srinophakun T, Chen HH, Chisti Y (2007) Optimization of lactic acid production by immobilized Lactococcus lactis IO-1. J Ind Microbiol Biotechnol 34:381–391
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Takagi M, Karseno YT (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Acknowledgments
This work was supported mainly by Walailak University, Thailand. At Kasetsart University, this work was supported by the Institute of Food Research and Product Development (IFRPD) and the Center of Advanced Studies for Tropical Natural Resources, Kasetsart University Institute for Advanced Studies (KUIAS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirisansaneeyakul, S., Singhasuwan, S., Choorit, W. et al. Photoautotrophic Production of Lipids by Some Chlorella Strains. Mar Biotechnol 13, 928–941 (2011). https://doi.org/10.1007/s10126-010-9355-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-010-9355-2