Abstract
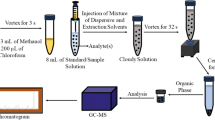
Quantitative determination by high performance liquid chromatography (HPLC) was performed for gymnodimine-A (GYM-A), a phycotoxin responsible for the contamination of Tunisian clams. This study demonstrates a rapid and reproducible HPLC-ultraviolet (UV) method for extraction, detection and quantification of GYM-A in toxic clams. The extraction of GYM-A from the digestive gland of clams in acetone, subsequent clean-up with diethyl ether and extraction with dichloromethane is the more valid protocol. Chromatography analyses were performed using a gradient of acetonitrile–water (10:90 to 90:10), containing trifluoroacetic acid (0.1%) for 20 min at 1 mL/min rate with a C18 column. Recovery rates exceeded 96%, and limits of detection and quantification were 5 ng/mL and 8 ng/g digestive gland, respectively. Repeatability and reproducibility were tested for various samples containing different levels of GYM-A. A significant correlation was observed between toxicity level of samples and the determined amount of GYM-A. Also, the persistence of GYM-A in contaminated clams from Boughrara lagoon was demonstrated. The kinetics discharge study of GYM-A in controlled medium, during 1 month, showed that the process of depuration was biphasic with an exponential discharge of 75% of the total amount of sequestered GYM-A during the first 12 days followed by a slow discharge (>10%) for the subsequent days up to the seventeenth day. This is the first time that a quantitative study of GYM-A in clams from Tunisian coasts is performed through the development of a new method for detection and quantify of this phycotoxin. We found HPLC-UV a reliable and suitable alternative to the mouse bioassay.






Similar content being viewed by others
References
Biré R, Krys S, Fremy JM, Dragacci S, Stirling D, Kharrat R (2002) First evidence on occurrence of gymnodimine in clams from Tunisia. J Nat Toxins 11:269–275
Bricelj VM, Shumway SE (1998) Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer Kinetics, and biotransformation. Rev Fish Sci 6:315–383
Chen CY, Chou HN (2001) Accumulation and depuration of paralytic shellfish poisoning toxins by purple clam Hiatula rostrata Lighttoot. Toxicon 39:1029–1034
Christian B, Luckas B (2008) Determination of marine biotoxins relevant for regulations: from the mouse bioassay to coupled LC-MS methods. Anal Bioanal Chem 391(1):117–134
Drira Z, Hamza A, Belhassen M, Ayadi H, Bouaïn A, Aleya L (2008) Dynamics of dinoflagellates and environmental factors during the summer in the Gulf of Gabes (Tunisia, Eastern Mediterranean Sea). Scientia Marina 72:59–71
European Community (2002) Commission decision of 15 March 2002 laying down detailed rules for the implementation of Council directive 91/492/EEC as regards the maximum levels and the methods of analysis of certain marine biotoxins in bivalve molluscs, echinoderms, tunicates and marine gastropods (2002/225/EC). Off J Eur Communities, L 75:62–64
Fux E, McMillan D, Bire R, Hess P (2007) Development of an ultra-performance liquid chromatography–mass spectrometry method for the detection of lipophilic marine toxins. J Chromatogr A 1157:273–280
Hashimoto S, Suzuki T, Shirota Y, Honma M, Itabashi Y, Chyounan T, Kamiyama T (2006) Lipophilic toxin profiles associated with diarrhetic shellfish poisoning in scallops, Patinopecten yessoensis, collected in Hokkaido and comparison of the quantitative results between LC/MS and mouse bioassay. J Food Hyg Soc Jpn 47:33–40
Haywood AJ, Steidinger KA, Truby EW, Bergquist PR, Bergquist PL, Adamson J, MacKenzie L (2004) Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J Phycol 40:165–179
Hess P, McMahon T, Slattery D, Swords D, Dowling G, McCarron M, Clarke D, Gibbons W, Silke J, O’Cinneide M (2003) Use of LC–MS testing to identify lipophilic toxins, to establish local trends and interspecies differences and to test the comparability of LC–MS testing with the mouse bioassay: an example from the Irish Biotoxin Monitoring Programme 2001. In: Villalba A, Reguera B, Romalde JL, Beiras R (eds) Molluscan shellfish safety. Xunta de Galicia and UNESCO, Spain, pp 57–66
Hu T, Curtis JM, Oshima Y, Quilliam MA, Walter JA, Watson-Wright WM, Wright JLC (1995) Spirolides B and D, two novel macrocycles isolated from the digestive glands of shellfish. J Chem Soc Chem Commun 20:2159–2161
Kharrat R, Servent D, Girard E, Ouanounou G, Amar M, Marrouchi R, Benoit E, Molgó J (2008) The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J Neurochem 21:952–963
Mackenzie L, Holland P, McNabb P, Beuzenberg V, Selwood A, Suzuki T (2002) Complex toxin profiles in phytoplankton and Green shell mussels (Perna Canaliculus), revealed by LC-MS/MS analysis. Toxicon 40:1321–1330
Miles CO, Wilkins AL, Stirling DJ, Mackenzie AL (2000) New analogue of gymnodimine from a Gymnodinium species. J Agric Food Chem 48:1373–1376
Miles CO, Wilkins AL, Stirling DJ, Mackenzie AL (2003) Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J Agric Food Chem 51(16):4838–4840
Oikawa H, Satomi M, Watabe S, Yano Y (2005) Accumulation and depuration rates of paralytic shellfish poisoning toxins in the shore crab Telmessus acutidens by feeding toxic mussels under laboratory controlled conditions. Toxicon 45:163–169
Powell CL, Doucette GJ (1999) A receptor binding assay for paralytic shellfish poisoning toxins: recent advances and applications. Nat Toxins 7:393–400
Seki T, Satake M, Mackenzie L, Kaspar HF, Yasumoto T (1995) Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dino-flagellate Gymnodinium sp. Tetrahedron Lett 36:7093–7096
Stirling DJ (2001) Survey of historical New Zealand shellfish samples for accumulation of gymnodimine. N Z J Mar Freshwater Res 35:851–857
Stobo LA, Lacaze JPCL, Scott AC, Petrie J, Turrell EA (2008) Surveillance of algal toxins in shellfish from Scottish waters. Toxicon 51:635–648
Takada N, Umemura N, Suenaga K, Uemura D (2001) Structural determination of the pteriatoxins A, B, and C, extremely potent toxins from the bivalve Pteria penguin. Tetrahedron Lett 42:3495–3497
Toyofuku H (2006) Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report). Mar Pollut Bull 52:1735–1745
Turrell EA, Stobo L (2007) A comparison of the mouse bioassay with liquid chromatography–mass spectrometry for the detection of lipophilic toxins in shellfish from Scottish waters. Toxicon 50:442–447
Uemura D, Chou T, Hainao T, Nagatsu A, Fukuzawa S, Zheng S-Z, Chen H-S (1995) Pinnatoxin A: a toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J Am Chem Soc 117:1155–1156
Wang Z, King KL, Ramsdell JS, Doucette GJ (2007) Determination of domoic acid in seawater and phytoplankton by liquid chromatography–tandem mass spectrometry J. Chromatogr A 1163:169–176
Yasumoto T, Oshima Y, Yamaguchi M (1978) Occurrence of a new type shellfish poisoning in the Tohoku district. Bull Jap Soc Sci Fish 44:1249–1255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marrouchi, R., Dziri, F., Belayouni, N. et al. Quantitative Determination of Gymnodimine-A by High Performance Liquid Chromatography in Contaminated Clams from Tunisia Coastline. Mar Biotechnol 12, 579–585 (2010). https://doi.org/10.1007/s10126-009-9245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9245-7