Abstract
Background
For patients with locally advanced proximal gastric cancer (LAPGC), the individualized selection of patients with highly suspected splenic hilar (No. 10) lymph node (LN) metastasis to undergo splenic hilar lymphadenectomy, is a clinical dilemma. This study aimed to re-evaluate the feasibility and safety of laparoscopic spleen-preserving splenic hilar lymphadenectomy (LSPSHL) and to identify the population who would benefit from it.
Methods
A total of 1068 patients (D2 group = 409; D2 + No. 10 group = 659) who underwent laparoscopic total gastrectomy from four prospective trials between January 2015 and July 2019 were analyzed.
Results
No significant difference in the incidence (16.9% vs. 16.4%; P = 0.837) of postoperative complications were found between the two groups. The metastasis rate of No. 10 LN among patients in the D2 + No. 10 group was 10.3% (68/659). Based on the decision tree, patients with LAPGC with tumor invading the greater curvature (Gre), patients with non-Gre-invading LAPGC with a tumor size > 5 cm and clinical positive locoregional LNs were defined as the high-priority No. 10 dissection group. The metastasis rate of No. 10 LNs in the high-priority group was 19.4% (41/211). In high-priority group, the 3-year overall survival of the D2 + No. 10 group was better than that of the D2 group (74.4% vs. 42.1%; P = 0.005), and the therapeutic index of No. 10 was higher than the indices of most suprapancreatic stations.
Conclusions
LSPSHL for LAPGC is safe and feasible when performed by experienced surgeons. LSPSHL could be recommended for the high-priority group patients even without invasion of the Gre.
Similar content being viewed by others
Introduction
The incidence of proximal gastric cancer (GC) has been gradually increasing globally [1,2,3]. Although radical total gastrectomy (TG) is still the preferred treatment modality for locally advanced proximal gastric cancer (LAPGC), whether it is essential to dissect the splenic hilar (No. 10) lymph nodes (LNs) is still controversial [4,5,6,7]. According to previous reports, the No. 10 LN metastatic rate for LAPGC was about 8.1–20.9% [8,9,10,11,12]. Thus, standard D2 lymphadenectomy with total gastrectomy should include No. 10 LNs as directed in the earlier versions of the “Japanese GC Treatment Guidelines” [4, 5]. Due to the high metastasis rate and high therapeutic index of the No. 10 LN for LAPGC on invading the greater curvature (Gre) [12,13,14], a splenic hilar lymphadenectomy (SHL) for these patients has been recommended by most surgeons. The latest treatment guidelines [7] proposed that a dissection effect of the No. 10 LN on tumors invading the Gre can be expected.
Traditionally, a splenectomy appears to be mandatory for dissection of the No. 10 LN. This special issue was emphasized in the 5th edition of the “Japanese GC Treatment Guidelines” (Clinical Question 4) [15]. A randomized controlled (JCOG 0110) trial [16] comparing spleen-preservation and splenectomy for LAPGC not invading the Gre (non-Gre) showed that the metastasis rate of the No. 10 LN was 2.7% in the splenectomy group and 3.5% in the spleen-preservation group. Moreover, splenectomy increases operative morbidity without improving survival. Accordingly, No. 10 LN was excluded from the D2 lymphadenectomy for LAPGC in the latest guideline [7]. However, the lower rate of No. 10 LN metastasis in the JCOG 0110 trial may be influenced by the fact that 68.5% of the enrolled patients were at stage I–II disease, and that it excluded gross LN metastasis along the splenic artery or splenic hilum. This does not fully reflect the regularity of metastasis and the dissection value of No. 10 LN. In addition to the cross-sectional position of the tumor, other factors such as larger tumor size, Borrmann type IV, and poorly differentiated tumor are also related to the metastasis of splenic hilar LNs [17,18,19,20]. Hence, it is arbitrary to deny SHL for all patients with non-Gre-invading LAPGC.
In addition to the LAPGC itself clinicopathological features, manner of dissection and corresponding safety will also affect the decision of the surgeon whether to perform SHL. Limited devices and techniques, and to thoroughly dissect splenic hilar LNs, early SHL often combined splenectomy [21, 22]. However, a series of studies have shown that compared with spleen-preserving TG, combined splenectomy increases operative morbidity and mortality without improving survival [16, 23, 24]. Therefore, spleen-preserving SHL (SPSHL) gradually causes concern; however, due to the special anatomy of the spleen and vascular complexity, open SPSHL still has great challenges. Because laparoscopic gastrectomy (LG) is more precise in the identification of the perigastric fascia, fascial space, blood vessels, and other structures during lymphadenectomy compared with open surgery, since Hyung [25] first reported on laparoscopic SPSHL (LSPSHL) in 2008, many surgeons suggest that LSPSHL is not only safe but also has the significant advantage of fast postoperative recovery compared with open surgery [6, 26,27,28]. A recent multicenter study [9] showed that the overall incidence of postoperative complication was 13.6%, and the major complication rate was 3.3% for patients undergoing LSPSHL, which suggested that compared to the high morbidity and mortality associated with splenectomy surgery, LSPSHL is safe and feasible in experienced medical centers.
In the current era of minimally invasive surgery, a high-level evidence-based medicine evaluation of the safety and efficacy of LSPSHL and correctly identifying the beneficial population from LSPSHL, could provide strong guidance to reduce the confusion in clinical practice. However, limited by available patients, time, and study design, it is difficult for a single prospective study to ideally weigh all clinical aspects and answer these questions. Therefore, we conducted this pooled analysis of four previous prospective trials to re-evaluate the indications of LSPSHL, so as to provide a reference for developing a sustainable clinical practice guideline.
Methods
Participants
Between January 2015 and July 2019, a total of 1491 patients were enrolled into four separate prospective trials. The CLASS-04 trial (ClinicalTrials.gov, NCT02845986) enrolled 251 patients between September, 2016 and October, 2017 to evaluate the safety and feasibility of LSPSHL for LAPGC, conducted at specialized institutions of the Chinese Laparoscopic Gastrointestinal Surgery Study group [9]. The FUGES-001 trial (ClinicalTrials.gov, NCT02327481) enrolled 438 patients between January, 2015 and April, 2016 to determine the efficiency of 3D LG and 2D LG in GC, conducted at Fujian Medical University Union Hospital (FMUUH) [29, 30]. The FUGES-002 trial (ClinicalTrials.gov, NCT02333721) enrolled 536 patients between January, 2015 and December, 2018 to evaluate the surgical outcomes of LSPSHL for non-Gre-invading LAPGC, conducted at FMUUH. The FUGES-012 trial (ClinicalTrials.gov, NCT03050879) enrolled 266 patients between November, 2018 and July, 2019 to investigate the efficacy of indocyanine green (ICG) tracer-guided lymphadenectomy during LG, conducted at FMUUH [31]. Each of the four studies were approved by their institution’s local ethics committee. Operative techniques, definitions of study endpoints, and results of these studies have been reported previously [9, 29,30,31]. Heterogeneity between the four trials was minimized by the use of the same laparoscopic surgery procedure.
All four prospective trials had similar inclusion and exclusion criteria, except for definite tumor location and clinical T (cT) category in each protocol. Patients who received neoadjuvant therapy were not included in our trials. Patients were eligible for this pooled analysis if they had undergone TG. We excluded the following patients: patients who withdrew consent, patients intraoperatively confirmed as unable to complete R0 resection due to tumor, patients who underwent partial gastrectomy, patients with tumors located in the lower third of the stomach, or those with tumors invading the esophagus. Pathologic evaluation of all resected specimens was performed according to a standardized manner [32]. All patients underwent the same perioperative management and follow-up protocol.
Procedures
The study population was divided into two groups according to the allocated procedures as follows [7].
D2 group
Laparoscopic total gastrectomy (LTG) with D2 lymphadenectomy. The lymphadenectomy extent of the D2 group included the Nos. 1, 2, 3, 4, 5, 6, 7, 8a, 9, 11p, 11d, and 12a LNs. Video S1 demonstrates the procedure of lymphadenectomy without No. 10 dissection in the splenic hilar area.
D2 + No. 10 group
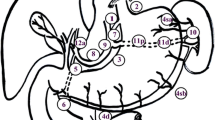
LTG with D2 plus LSPSHL. The lymphadenectomy extent of the D2 + No. 10 group included the Nos. 1, 2, 3, 4, 5, 6, 7, 8a, 9, 10, 11p, 11d, and 12a LNs. No. 10 LN included those adjacent to the splenic artery distal to the pancreatic tail, those on the roots of the short gastric arteries, and those along the left gastroepiploic artery proximal to its first gastric branch [32]. During lymphadenectomy, the pancreas and the spleen were not mobilized. The left gastroepiploic artery was ligated and cut at the origin, LNs along the splenic artery and at the hilum of the spleen were dissected without sacrificing the spleen and splenic vessels [33]. Video S2 demonstrates the LSPSHL procedure.
Figure S1 shows the intraoperative view of the splenic hilar after D2 and D2 + No. 10 lymphadenectomy.
Surgical quality control
All surgeons participating in the four prospective trials met the minimum requirements of having performed more than 50 cases of LTG prior to the trials.
To confirm the rationale for the surgical procedure, the quality of D2 LN dissections, and the integrity of the specimens, a series of photographs obtained during surgery and an unedited video of the laparoscopic operation in the four trials were saved for assessment through a sample survey for standardization and quality control (Table S1).
Definitions
The cT category and clinical N (cN) category of disease was determined according to the seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual [34]. Locoregional nodes are suspicious for tumor involvement (cN+) if round and/or > 8 mm in short axis diameter in preoperative imaging [35,36,37].
LAPGC was defined as tumor in the upper or middle third part of stomach with cT2-4aN0-3M0 stage at preoperative evaluation [34]. The stomach’s cross-sectional circumference is divided into four equal parts [32]: the lesser curvature (Less) and Gre, and the anterior (Ant) and posterior (Post) walls (Figure S2). Macroscopic and pathologic images were reviewed to determine whether the tumors invaded the Gre or not. “Non-Gre” including Less, Ant, Post. Circumferential involvement is classified as Gre.
The metastasis rate was calculated using the number of patients who underwent dissection of the station as the denominator and the number of patients who had pathological metastasis in the station as the numerator. All LNs were classified into 3 categories according to the metastasis rate [38], as follows: category-1 nodes (strongly recommended for dissection), for metastasis rates exceeding 10%; category-2 nodes (weakly recommended for dissection), for metastasis rates between 5% and 10%; and category-3 nodes (not recommended for dissection), for metastasis rates less than 5%.
Morbidity and mortality were assessed within 30 days after surgery. Postoperative complications were graded according to the Clavien–Dindo classification [39].
Survival analysis
Three of the four included studies (CLASS-04, FUGES-002, and FUGES -012) have not reached the actual follow-up time of 3 years, so to ensure the accuracy and reliability of survival analysis, the survival data of patients in the FUGES-001 trial were used for exploratory analysis. The cutoff follow-up date for the analysis of the FUGES-001 trial was April 2020, by which time all living patients had reached a minimum of 3 years of follow-up. Overall survival (OS) was defined as the time from surgery to death from any cause. The life-table method was used to calculate the 5-year OS. We adopted the therapeutic value index to evaluate the efficacy of nodal dissection [11]. The index was calculated by multiplying the metastasis rate of the station and the 5-year OS of patients with metastasis to that station.
Statistical analysis
Data were analyzed using the intention-to-treat principle. For bias reduction in the comparison of a treatment to a non-randomized control group, propensity score methods (PSM) were used to further evaluate the surgical outcome and postoperative recovery between D2 group and D2 + No. 10 group [40]. The propensity scores were calculated using a logistic regression model with the following covariates: age, sex, body mass index (BMI), Eastern Cooperative Oncology performance status, cT category, and cN category. We imposed a calliper of 0.005 of the standard deviation of the logit of the propensity score. The cut off value of an absolute standardized mean difference above which a meaningful imbalance is indicated was 0.100 [41, 42].
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as numbers. The differences between the groups were assessed using a t test or χ2 test, as appropriate. Survival was calculated using the Kaplan–Meier method, and differences between groups were compared using a log-rank test. A Cox proportional hazards regression model was used to determine the independent prognostic factors associated with OS. Variables with a value of P < 0.05 in the univariate analysis were subsequently included in a multivariate Cox proportional hazards regression. Stepwise backward variable removal was applied to the multivariate model. All tests were two-sided with the significance level set at P < 0.05. All data were analyzed using R software (version 3.6.1).
Results
Baseline characteristics
Of the 1491 original patients, 1068 were eligible for inclusion in this pooled analysis (Fig. 1). A total of 409 patients were assigned to the D2 group, and 659 patients were assigned to the D2 + No. 10 group. The general characteristics of the two groups before PSM are shown in Table 1. The total mean (SD) number of retrieved LNs (RLNs) in the D2 + No. 10 group was 43.8 (16.2), which was significantly higher than that in the D2 group [40.5 (13.6); P < 0.001]. After subtracting the number of No. 10 RLNs, the number of RLNs was comparable between the D2 + No. 10 and D2 groups (40.9 vs. 40.5; P = 0.708). Before matching, there were significant difference in the distribution of BMI, tumor size, histology, pN category, and AJCC 8th staging. After matching, the remaining 562 patients, 281 in the D2 group and 281 in the D2 + No. 10 group, were matched (Table 1). The preoperative clinicopathological features were comparable between the two groups (SMD all < 0.100). The total mean number of RLNs in the D2 + No. 10 group was significantly more than that in the D2 group (43.8 vs. 40.4; P = 0.005). The mean number of No. 10 RLNs was 2.7 in the D2 + No. 10 group after matching.
Surgical outcome and postoperative recovery
Before matching, compared with the D2 group, the D2 + No. 10 group had a longer surgical time (183.1 min vs. 215.9 min; P < 0.001) and the trend of more intraoperative blood loss (63.3 mL vs. 76.9 mL; P = 0.055). The postoperative recovery process was comparable (Table 2), except the D2 + No. 10 group had a longer time to first liquid intake than the D2 group (4.6 days vs. 5.3 days; P = 0.019). No significant difference was found between the two groups in the incidence of intraoperative complication (2.0% vs. 2.9%, P = 0.348). However, seven cases of splenic injury occurred in the D2 + No. 10 group, which was significantly more than occurred in the D2 group (P = 0.037). There was no significant difference in the incidence (16.9% vs. 16.4%; P = 0.837) and severity (P = 0.395) of postoperative complication between the two groups. An analysis of risk factors for postoperative complication (Table S2) found that older age, higher BMI, and poor performance status were independent risk factors for complication, and that D2 + No. 10 dissection was not associated with complication (P = 0.837).
After matching, the mean surgical time of the D2 + No. 10 group was 35 min longer than that of the D2 group (217.7 v 183.0 min; P < 0.001). The time to first liquid intake was significantly shorter in the D2 group than in the D2 + No. 10 group (P = 0.023). There was no significant difference in the incidence (14.9% vs. 17.8%; P = 0.362) and severity (P = 0.160) of postoperative complication between the two groups (Table 2).
No. 10 LN metastasis
Of 659 patients undergoing SHL, the mean number of No. 10 RLNs was 2.9, with a mean of 0.2 determined to be metastatic LNs. We then estimated the LN metastasis rate for all patients (n = 1068) at each station and the No. 10 LN metastasis rate in the D2 + No. 10 group (Table S3 and Figure S3). The metastasis rate of No. 10 LNs was 10.3% (68/659) in patients undergoing SHL. Subgroup analysis according to disease stage showed that the No. 10 LN metastasis rate was 4.8% (4/84) and 11.1% (64/575) in early GC and advanced GC, respectively. Subgroup analysis according to each cross-sectional part showed that the metastasis rate was 19.8% (17/86) if the tumor invading the Gre.
Comparing the metastasis rates of the No. 10 station and other stations (Figure S4), we found that the metastasis rate of No. 10 was similar to those of Nos. 6, 8a, and lower than those of Nos. 1, 2, 3, 4, 7, 9, and 11p, but exceeded those of Nos. 5, 11d, and 12a.
Risk factors for No. 10 LN metastasis
Univariate analysis of No. 10 LN metastasis (Table S4) using preoperative factors showed that tumor size, cross-sectional part, cT category, and cN category were related to No. 10 LN metastasis (all P < 0.05). Multivariate analysis showed that tumor size > 5 cm (P < 0.001), Gre (P = 0.019), and cN + (P = 0.003) were risk factors for No. 10 LN metastasis.
Based on the decision tree (Fig. 2), we found that the metastasis rate of No. 10 LNs was 3.7% (6/162) for patients with tumors located in the non-Gre of the stomach, < 5 cm, and cN0, which were classified as category-3 nodes. The metastasis rate of No. 10 LNs for patients with tumors in the non-Gre, ≤ 5 cm, and cN+ was 7.4% (19/258), which were category-2 nodes. And the metastasis rate of No. 10 LNs for patients with tumors in the non-Gre, > 5 cm, and cN0 was 7.1% (2/28), which were category-2 nodes. And the metastasis rate of No. 10 LNs for patients with tumors in the non-Gre, > 5 cm, and cN+ was 19.2% (24/125), which were category-1 nodes.
Based on the above results, patients with non-Gre-invading LAPGC with a tumor size ≤ 5 cm and patients with non-Gre-invading LAPGC with a tumor size > 5 cm and cN0 were defined as the low-priority No. 10 dissection group. Patients with LAPGC with tumor invading the Gre and patients with non-Gre-invading LAPGC (tumor size > 5 cm and cN+) were defined as the high-priority No. 10 dissection group. Figure 3 shows the metastasis rates of each station according to the No. 10 dissection priority. The No. 10 LN metastasis rate in the low-priority group was 6.0% (27/448), considered category-2 nodes, whereas Nos. 5, 6, 11d and 12a LNs were classified as nodes not recommended for dissection. The No. 10 LN metastasis rate in the high-priority group was 19.4% (41/211), classified as category-1 nodes, which were strongly recommended for dissection.
Regardless of whether low-priority or high-priority, no significant difference was found in the rates of intraoperative complication or postoperative complication (Table S5) in the D2 + No. 10 and D2 groups (all P > 0.05).
Survival benefit analysis of LSPSHL in the FUGES-001 trial
For 164 eligibility patients in the FUGES-001 trial, the median follow-up time was 52 months. The baseline characteristics of participants were balanced between the D2 and D2 + No. 10 groups, regardless of being low-priority or high-priority (Table S6). For patients in the low-priority group, the 3-year OS of the D2 and D2 + No. 10 groups were similar (82.9% vs. 85.1%; P = 0.801; Fig. 4). Univariate and multivariate Cox regression analysis of patients in the low-priority group showed that LSPSHL was not associated with OS [D2 + No. 10 vs. D2, hazard ratio (HR): 1.12; P = 0.801] (Table S7). For patients in the high-priority group, the 3-year OS of the D2 + No. 10 group was 74.4%, significantly greater than the 42.1% of the D2 group (P = 0.005). Multivariate Cox regression analysis of patients in the high-priority group showed that advanced pT category (pT4 vs. ≤ pT3, HR: 4.62; P < 0.001) was a risk factor for OS, and LSPSHL (D2 + No. 10 vs. D2, HR: 0.43; P = 0.026) was a protective factor for OS. Subgroup analysis of patients in the high-priority group (Figure S5) showed that the OS was comparable for patients with stage I–II disease, and the OS of patients with stage III disease who underwent SHL was better than that of patients without SHL (3-year OS: 64.5% vs. 38.9%; P = 0.055), although it did not reach statistical significance.
For patients who underwent SHL (n = 110), there was no significant difference in the 3-year OS of patients with positive No. 10 LN and that of patients negative No. 10 LN (75.0% vs. 81.4%; P = 0.877) (Figure S6). Univariate and multivariate Cox regression analysis revealed that advanced stage and perineural invasion were independent factors of OS, and that No. 10 LN metastasis was not the influencing factor (Table S8). Univariate Cox regression analysis of patients in the high-priority group that underwent SHL identified that the No. 10 LN metastasis was not the influencing factor for OS (Table S9).
For patients who underwent SHL, the therapeutic value index (Table 3) of the No. 10 was 1.5 in low-priority group and 11.6 in high-priority group, respectively. The index of station No. 10 in high-priority group ranked fifth, just below those of peri-gastric stations (Nos. 1, 2, 3, 4) and station No. 7. Remarkably, the index of station No. 10 was higher than the indices of the other suprapancreatic stations (Nos. 8a, 9, 11p, 11d, 12a). However, the index of station No. 10 in the low-priority group was just higher than those of Nos. 5 and 11d.
Discussion
In patients with GC, the metastasis rate of a specific LN station is an important index to evaluate the necessity of lymphadenectomy for this station [11, 38]. In this study, the metastasis rate of the No. 10 LN in clinical LAPGC (cT2–cT4a) was 10.3%. The metastasis rate of LN in the splenic hilar area is higher than that in the suprapyloric area nodes and as high as those in the infrapyloric area nodes and partial suprapancreatic area nodes. It is suggested that SHL may be suitable for some patients with LAPGC. Decision tree analysis showed whether the tumor invaded the Gre, whether the tumor size was > 5 cm, and whether preoperative locoregional LNs are suspicious for tumor involvement can be used in combination to evaluate the metastasis of No. 10 LN. Maezawa et al. [13] reviewed 82 patients with LAPGC that invaded the Gre. The metastatic rate of No. 10 LN was relatively high at 13%, and the therapeutic index was as high as that of other suprapancreatic nodes. Previous studies have shown that tumor size can affect metastasis of the splenic hilar LN [43, 44]. Patients should be considered to have a high metastasis rate of splenic hilar LN for larger LAPGC. Studies have also shown that LN metastasis in other stations can affect the metastasis of No. 10 LNs [17, 43]. The No. 10 LN metastasis rate of high-priority patients in the current study was 19.4%, which is much higher than 10%, indicating that they are strongly recommended for dissection.
The survival benefit analysis of No. 10 dissection showed that SPSHL was a protective factor for the OS of patients considered high-priority. For other patients with LAPGC, SHL did not improve the prognosis. In the high-priority group, the therapeutic value index of No. 10 came next to the perigastric nodes and was substantially higher than those of most suprapancreatic stations, all of which had been essential components of D2 dissection. This finding suggests that the priority of No. 10 is next to that of perigastric nodes and is higher than that of the lymph nodes along the splenic artery. Meantime, exploratory analysis showed that for patients in the high-priority group with LSPSHL, whether or not they had No. 10 LN metastasis did not affect the prognosis, which proved the effectiveness of LSPSHL for patients considered high-priority.
Regarding the safety of LSPSHL, the combined effort of more than 20 surgeons showed that on the premise of mastering the skills of LSPSHL, compared with D2 lymphadenectomy, although LSPSHL increases the operative time and potentially increases the intraoperative blood loss and the possibility of spleen injury, it does not increase the incidence of postoperative complication and postoperative mortality. Thus, experienced surgeons could carry out LSPSHL more safely and effectively according to their own operative characteristics [45,46,47].
We created the LSPSHL recommended flow chart for LAPGC (Fig. 2). SHL is not recommended for patients with non-Gre-invading LAPGC with a tumor size ≤ 5 cm and patients with non-Gre-invading LAPGC with a tumor size > 5 cm and cN0 to avoid unnecessary trauma and extend the lymphadenectomy. However, for patients with Gre tumors without infiltration of the spleen and splenic vessels, and some non-Gre tumors (size > 5 cm and cN+), LSPSHL could prove effective. And our evidence showed that LSPSHL is also safe and feasible for such patients. Therefore, we recommend that for patients with LAPGC with high-priority SHL, No. 10 LNs should be included in the routine range of D2 lymphadenectomy for TG.
This study collected data from four independent prospective trials with similar inclusion criteria, treatment methods, and postoperative management schedule, which greatly enhanced the level of evidence-based medicine. To the best of our knowledge, this study is the largest study investigating LSPSHL. However, there are still some limitations. First, although the pooled study provides us with a significant amount of real-world data to evaluate the feasibility and selectivity of LSPSHL, the four independent clinical studies of laparoscopic gastric surgery have their own research focus. For instance, in the FUGES-001 trial using 2D or 3D laparoscopic equipment and ICG-guided lymphadenectomy was used in the FUGES-012 trial. However, according to the stratified analysis of these factors that may affect the LSPSHL, there was no difference in the rate of No. 10 LN metastasis and postoperative complication between the experiment group and control group in both trials (Figure S7). Second, the surgeons enrolled in these studies were surgeons with rich experience in laparoscopic surgery. Because of the deep location of the splenic hilar area, the variability of the splenic vessels, and the complicated adjacent relationship, it is difficult for newly trained surgeons to perform LSPSHL. However, with the gradual development of medical specialization, more and more gastric surgeons have performed an exploration of LSPSHL. After surmounting the learning curve, we believe that this surgery, previously thought to be a sophisticated surgery, can also be popularized [48, 49]. Patients considered to be high-priority will therefore not lose the opportunity for radical treatment due to technical difficulties. Third, it is undeniable that not all patients have an actual 5-year follow-up, which will have an impact on the calculation of the therapeutic value index, as previous studies [13, 50]. Fourth, our exploratory analysis showed that LSPSHL could improve the OS of patients in the high-priority group without increasing the incidence of postoperative complication. However, due to the relatively limited number of cases, the long-term oncological effect of LSPSHL requires further follow-up.
In conclusion, this pooled analysis of four prospective trials showed that it is safe and feasible for experienced surgeons to perform LSPSHL. Similar to the guidelines, the current study does not recommend LSPSHL for all patients with proximal gastric cancer. We recommend LSPSHL for patients with LAPGC that are considered high-priority, including all patients with invasion into the Gre, and some patients with non-Gre-invading LAPGC (tumor size > 5 cm and with preoperative positive locoregional LNs).
References
Liu K, Yang K, Zhang W, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg. 2016;263:88–95.
Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manage Res. 2018;10:239–48.
Pastina M, Menna C, Andreetti C, Ibrahim M. The esophagogastric junctional adenocarcinoma an increasing disease. J Thorac Dis. 2017;9:1455–8.
Association JGC. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Association JGC. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Ma Z, Shi G, Chen X, et al. Laparoscopic splenic hilar lymph node dissection for advanced gastric cancer: to be or not to be. Ann Transl Med. 2019;7:343.
Association JGC. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020.
Ikeguchi M, Kaibara N. Lymph node metastasis at the splenic hilum in proximal gastric cancer. Am Surg. 2004;70:645–8.
Zheng CH, Xu YC, Zhao G, et al. Safety and feasibility of laparoscopic spleen-preserving No. 10 lymph node dissection for locally advanced upper third gastric cancer: a prospective, multicenter clinical trial. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-07306-8.
Jeong O, Jung MR, Ryu SY. Clinicopathological features and prognostic impact of splenic hilar lymph node metastasis in proximal gastric carcinoma. Eur J Surg Oncol. 2019;45:432–8.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–51.
Watanabe M, Kinoshita T, Enomoto N, et al. Clinical significance of splenic hilar dissection with splenectomy in advanced proximal gastric cancer: an analysis at a single institution in Japan. World J Surg. 2016;40:1165–71.
Maezawa Y, Aoyama T, Yamada T, et al. Priority of lymph node dissection for proximal gastric cancer invading the greater curvature. Gastric Cancer. 2018;21:569–72.
Yura M, Yoshikawa T, Otsuki S, et al. The therapeutic survival benefit of splenic hilar nodal dissection for advanced proximal gastric cancer invading the greater curvature. Ann Surg Oncol. 2019;26:829–35.
Komatsu S, Otsuji E. Essential updates 2017/2018: recent topics in the treatment and research of gastric cancer in Japan. Ann Gastroenterol Surg. 2019;3:581–91.
Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277.
Shin SH, Jung H, Choi SH, et al. Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol. 2009;16:1304.
Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89–92.
Sasada S, Ninomiya M, Nishizaki M, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res. 2009;29:3347–51.
Kosuga T. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer. 2011;14:172–7.
Yamamoto M, Baba H, Kakeji Y, et al. Postoperative morbidity/mortality and survival rates after total gastrectomy, with splenectomy/pancreaticosplenectomy for patients with advanced gastric cancer. Hepatogastroenterology. 2004;51:298–302.
Wanebo HJ, Kennedy BJ, Winchester DP, et al. Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on longterm survival. J Am Coll Surg. 1997;185:177–84.
Csendes A, Burdiles P, Rojas J, et al. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–7.
Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2010;93:559–63.
Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6.
Sakuramoto S, Kikuchi S, Futawatari N, et al. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416–23.
Guo X, Peng Z, Lv X, et al. Randomized controlled trial comparing short-term outcomes of laparoscopic and open spleen-preserving splenic hilar lymphadenectomy for advanced proximal gastric cancer: an interim report. J Surg Oncol. 2018;118:1264–70.
Hosogi H, Okabe H, Shinohara H, et al. Laparoscopic splenic hilar lymphadenectomy for advanced gastric cancer. Transl Gastroenterol Hepatol. 2016;1:30.
Zheng CH, Lu J, Zheng HL, et al. Comparison of 3D laparoscopic gastrectomy with a 2D procedure for gastric cancer: a phase 3 randomized controlled trial. Surgery. 2018;163:300–4.
Lu J, Xu BB, Zheng ZF, et al. Does three-dimensional surgery affect recurrence patterns in patients with gastric cancer after laparoscopic R0 gastrectomy? Results from a 3-year follow-up phase III trial. Surg Endosc. 2020. https://doi.org/10.1007/s00464-020-07367-0.
Chen QY, Xie JW, Zhong Q, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. 2020;155:300–11.
Association JGC. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101.
Huang CM, Chen QY, Lin JX, et al. Huang’s three-step maneuver for laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer. Chin J Cancer Res. 2014;26:208–10.
Frederick LGMD, David LPMD, Irvin DFMD, et al. AJCC cancer staging manual. Berlin: Springer; 2010.
Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–22.
Kim HJ, Kim AY, Oh ST, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879–85.
Zheng ZF, Lu J, Zheng CH, et al. A novel prognostic scoring system based on preoperative sarcopenia predicts the long-term outcome for patients After R0 resection for gastric cancer: experiences of a high-volume center. Ann Surg Oncol. 2017;24:1795–803.
Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000003499.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–84.
Shin HJ, Son SY, Wang B, et al. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2084 consecutive patients. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000003845.
Li C, Kim S, Lai JF, et al. Lymph node dissection around the splenic artery and hilum in advanced middle third gastric carcinoma. Eur J Surg Oncol. 2009;35:709–14.
Sun Z, Wang Q, Yu X, et al. Risk factors associated with splenic hilar lymph node metastasis in patients with advanced gastric cancer in northwest China. Int J Clin Exp Med. 2015;8:21358–64.
Okabe H, Obama K, Kan T, et al. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1–6.
Huang CM, Chen QY, Lin JX, et al. Laparoscopic spleen-preserving no. 10 lymph node dissection for advanced proximal gastric cancer using a left approach. Ann Surg Oncol. 2014;21:2051.
Zheng L, Zhang C, Wang D, et al. Laparoscopic spleen-preserving hilar lymph node dissection through pre-pancreatic and retro-pancreatic approach in patients with gastric cancer. Cancer Cell Int. 2016;16:52.
Huang ZN, Huang CM, Zheng CH, et al. Learning curve of the application of Huang three-step maneuver in a laparoscopic spleen-preserving splenic hilar lymphadenectomy for advanced gastric cancer. Medicine. 2016;95:e3252.
Huang CM, Huang ZN, Zheng CH, et al. Huang’s three-step maneuver shortens the learning curve of laparoscopic spleen-preserving splenic hilar lymphadenectomy. Surg Oncol. 2017;26:389–94.
Kano Y, Ohashi M, Ida S, et al. Therapeutic value of splenectomy to dissect splenic hilar lymph nodes for type 4 gastric cancer involving the greater curvature, compared with other types. Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01072-6.
Funding
Scientific and Technological Innovation Joint Capital Projects of Fujian Province (2017Y9011, 2017Y9004, 2018Y9041). Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171). The Second Batch of Special Support Funds for Fujian Province Innovation and Entrepreneurship Talents (2016B013). National Natural Science Foundation of China (No. 81802312). China Scholarship Council (201908350095). Special Fund for Clinical Research of Wu JiePing Medical Foundation (No. 320.6750.17511).
Role of the funder/sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Access to data and data analysis
Huang CM and Li P had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional contributions
We are grateful to the Chinese Gastric Cancer Association, the Chinese Society of Laparo-Endoscopic Surgery, and the Chinese Society of Gastrointestinal Surgery for their scientific support. We thank who have devoted a lot to this study, including nurses, pathologists, further-study doctors, statisticians, reviewers and editors. Especially Mr. Lei Lin. They were not financially compensated for their contributions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1.
Intraoperative view of the splenic hilar after the D2 (A) and D2 + No. 10 (B) LN dissection. Figure S2. Pattern diagram, the four equal parts of the gastric circumference. Less: lesser curvature; Gre: greater curvature; Ant: anterior wall; Post: posterior wall. Figure S3. Metastasis rates of each station (A) in early stage patients (pT1); (B) in advanced stage patients (pT2–pT4a). Figure S4. Comparison of the metastasis rates of No. 10 LNs and other stations (A) in all patients; (B) in advanced stage patients. Figure S5. Kaplan–Meier survival curves of the D2 and D2 + No. 10 groups for patients with stage I–II (A) and stage III (B) disease in the high-priority group. Figure S6. Kaplan–Meier survival curves of the No. 10(+) and No. 10(−) groups for patients who underwent D2 + No. 10 dissection. Figure S7. The No. 10 metastasis rate and postoperative complication rate of 2D vs. 3D groups and ICG vs. non-ICG groups for patients who underwent D2 + No. 10 lymph node dissection. Table S1. Checklist for determining the success of D2 lymphadenectomy. Table S2. Univariate and multivariate logistic regression analysis of postoperative complications. Table S3. Metastasis rates of each station in patients with proximal gastric cancer. Table S4. Risk factor analysis of the No. 10 lymph node metastasis in patients who underwent laparoscopic spleen-preserving splenic hilar lymphadenectomy (n = 659). Table S5. Postoperative complications and intraoperative complications for the D2 and D2 + No. 10 groups according to the No. 10 dissection priority. Table S6. Basic characteristics of the D2 and D2 + No. 10 groups in patients considered low-priority and high-priority in the FUGES-001 Trial. Table S7. Univariate and multivariate analyses for overall survival in patients in the high-priority and low-priority No. 10 dissection groups in the FUGES-001 Trial. Table S8. Univariate and multivariate analyses for overall survival in patients who underwent D2 + No. 10 LN dissection in the FUGES-001 Trial (n = 110). Table S9. Univariate analyses for overall survival in patients in the high-priority No. 10 dissection group who underwent D2 + No. 10 LN dissection in the FUGES-001 trial (n = 43).
Video S1. The D2 lymph node dissection procedure in the splenic hilar area.
Video S2. The laparoscopic spleen-preserving No. 10 lymph node dissection procedure in the splenic hilar area.
Rights and permissions
About this article
Cite this article
Zhong, Q., Chen, QY., Xu, YC. et al. Reappraise role of No. 10 lymphadenectomy for proximal gastric cancer in the era of minimal invasive surgery during total gastrectomy: a pooled analysis of 4 prospective trial. Gastric Cancer 24, 245–257 (2021). https://doi.org/10.1007/s10120-020-01110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01110-3