Abstract
Background
Little is known about cytokine and angiogenic factors (CAFs) in gastric cancer (GC) in terms of tumor classification and prognostic value. Here, we aimed to correlate CAF signature with overall survival (OS) in GC.
Methods
We measured pretreatment serum levels of 52 kinds of CAFs in 68 GC patients who were treated with fluoropyrimidine and platinum combination chemotherapy using multiplex bead immunoassays and enzyme-linked immunosorbent assay. We evaluated correlations between CAF levels and pathological features and OS.
Results
Three distinct patient groups were identified: one with high levels of proangiogenic factors, another with high levels of proinflammatory factors, and the other with high levels of both factors. Eleven CAFs [interleukin (IL)-2 receptor-alpha, growth-regulated alpha protein, hepatocyte growth factor, macrophage colony-stimulating factor, stromal cell-derived factor, IL-6, IL-8, IL-10, interferon-gamma, vascular endothelial growth factor, and osteopontin] were independently correlated with poor OS. Clustering analysis of these 11 CAFs revealed distinct high and low 11-CAF signature groups. High 11-CAF signature was associated with shorter OS (10.1 vs. 17.9 months, p = 0.026) along with poor performance status, and the presence of signet ring cell components in multivariate analysis of OS (HR 1.76, p = 0.029). The patients’ traditional clinicopathological characteristics were not significantly different between the high and low 11-CAF signature groups.
Conclusion
Serum CAF profiling differentiated GC patient groups. A high 11-CAF signature could identify GC patients with a poor prognosis when treated with standard chemotherapy who need urgent new treatment strategies.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the third leading cause of cancer death worldwide [1]. Metastatic or recurrent GC has a median overall survival (OS) of approximately 1 year, if treated with cytotoxic chemotherapy [2]. Recent advances in genomic approaches have revealed that GC is not a single disease, but can be classified as four subtypes: EBV-positive, microsatellite instability, genomically stable, and chromosomal instability [3]. Also, recurrent amplification of genes associated with the receptor tyrosine kinase pathway, such as EGFR, ERBB2, ERBB3, JAK2, FGFR2, and MET, and the angiogenesis pathway, such as VEGFA, have been reported in GC [4, 5]. With the exception of a subset of patients with ERBB2 amplification who experienced benefits from trastuzumab [6], targeted agents for aberrant gene amplification have not yet been successful in therapeutic applications [7–9].
Growing evidence shows that not only the tumor itself but also its niche and inflammatory cytokines are important considerations when defining a tumor [10, 11]. Moreover, recent successes in clinical trials that studied immune checkpoint inhibitors provided support for the new anti-tumor strategy of immune modulation [12, 13]. Comprehensive genomic research in GC has already shown that a subset of EBV-positive GC exhibited immune evasion mechanisms by amplification of programmed death ligand 1 (PD-L1 or CD274) [3], and recent data have shown promising results of PD-1 inhibition in GC [14]. The development of the optimal anti-tumor immunological strategy should begin with an exploration of immunological factors, such as cytokines and angiogenic factors (CAFs), which are associated with the tumor. To understand the immunological landscape of a tumor, subgroups have been defined on the basis of circulating CAFs and their clinical implications have been explored in solid tumors [15–19]. In renal cell carcinoma, 6 CAFs that were predefined as the “angiogenic group” were also identified as significant predictive factors for adding interferon-alpha treatment to sorafenib [16]. Moreover, levels of CAFs that were known as a “hypoxia signature” were higher in patients who experienced progression after induction chemotherapy in head and neck cancer [18]. In these studies, the used CAFs were various in terms of item and number. The CAFs work in the context of complex network. An understanding of CAF networks has been significantly correlated with clinical outcomes in several cancers, but little has been studied in GC. In different tumor types, the involved CAF networks might be different.
In this study, serum levels of all available CAFs were analyzed with clinicopathological features and clinical outcomes of GC patients. We hypothesized that patients with GC could be identified by distinct groups based on the serum CAF profile. Moreover, we aimed to find the key CAF signature that was significantly correlated with OS of GC patients treated with conventional chemotherapy.
Patients and methods
Patients
This study was a retrospective analysis of de-identified patient-level data collected from medical charts. Patients diagnosed with GC at Seoul National University Hospital, Republic of Korea, from April 2005 to December 2011 were included in the analysis if they were older than 18 years of age and had histologically confirmed recurrent or metastatic GC, an Eastern Cooperative Oncology Group performance status of 0–2, adequate organ function, and received chemotherapy with fluoropyrimidine and platinum combination.
Sample preparation and CAF analysis
Patients provided written informed consent for the collection of blood samples for biomarker analysis. Specimens were obtained before initiation of palliative chemotherapy. A total of 52 CAFs present in serum were analyzed according to the manufacturers’ instructions with multiplex bead suspension array kits using the Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA, USA), including human group I and II cytokine panels as described in previous reports [15, 16]. Serum concentrations of soluble carbonic anhydrase IX (sCA9), soluble vascular endothelial growth factor receptor-2, placental growth factor, and osteopontin (OPN) were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). Each serum sample was analyzed in duplicate and mean CAF concentrations were reported in pg/ml. Analytes for which more than 50 % of patients had nondetectable levels or coefficients of variation greater than 20 % were not included in the subsequent analyses, based on the previous reports of CAF analysis [16, 18]. Analytes with nondetectable levels were recorded as one-half of the lower threshold value.
Statistical analysis
The primary objective of this study was to determine whether pretreatment serum CAF levels correlated with clinicopathological characteristics of GC and clinical outcomes such as OS of patients with GC who were treated with conventional chemotherapy. The CAF concentrations analyzed in the study were log transformed because concentrations were highly skewed in all samples. For unsupervised hierarchical clustering, the log-transformed concentration of each baseline CAF was standardized by subtracting the sample mean and dividing by the standard deviation. Hierarchical clustering and data presentation were performed with Cluster 3.0 and TreeView v. 1 software (downloaded from http://www.eisenlab.org/). Wilcoxon rank-sum tests were applied to compare the differences of continuous variables between the groups.
OS was measured from the first day of palliative chemotherapy until death or the last follow-up date, if censored. Progression-free survival (PFS) was measured from the first day of palliative chemotherapy until disease progression or final follow-up visit and to date of disease progression confirmed by imaging modality. To identify the optimal cutoff of CAF levels to predict survival outcomes, differences in OS of binary groups according to each CAF level were compared and optimal cutoffs were determined by the lowest p value of the log-rank test [20]. All p values were two sided, and p < 0.05 was considered statistically significant. Analyses were completed with STATA version 12 software (StataCorp LP, College Station, TX, USA).
Ethics
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (H-1411-022-623). The study was conducted according to guidelines for biomedical research outlined in the Declaration of Helsinki.
Results
Patient characteristics
Of the 68 GC patients enrolled in the study, 40 patients received combination chemotherapy with 5-fluorouracil and oxaliplatin (FOLFOX) and 28 patients received capecitabine and cisplatin (XP) as first-line palliative chemotherapy. Table 1 shows the general clinicopathological characteristics of the 68 GC patients who were included in the final study analysis. Seven patients (10.3 %) were HER2 positive and 16 patients had diffuse-type disease, according to the Lauren classification system [21]. Of 22 patients (32.4 %) who had signet ring cell components, 13 had pure poorly cohesive carcinoma. The median follow-up duration was 81.6 months (range, 32.6–113 months), and the median OS and progression-free survival (PFS) of first-line palliative chemotherapy were 12.1 and 6 months, respectively. Clinicopathological characteristics were not significantly different between the two chemotherapy regimens (FOLFOX and XP; data not shown).
Unsupervised hierarchical clustering by CAF concentration
A total of 52 CAFs were initially measured and analyzed, but 10 CAFs were excluded from the final analysis because more than half the samples were outside the detection range. The mean, standard error, median, and range of the 52 CAFs are listed in Table 2.
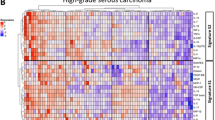
Unsupervised hierarchical clustering identified three groups of patients (Fig. 1). Half the patients were characterized by relatively high concentrations of proangiogenic and hypoxia-regulated factors (angiogenic group, N = 34), including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), macrophage colony-stimulating factor (M-CSF), growth-regulated alpha-protein (GRO-α), and stromal-derived factor 1-alpha (SDF-1α). The second group of patients (inflammatory group, N = 17) included patients with high levels of interleukins (ILs) and other proinflammatory factors. The third group of patients (dual group, N = 17) had elevated levels of both angiogenic and inflammatory factors. Clinicopathological features were not significantly different among the three patient groups (Supplementary Table 1). Survival analysis showed that patients who had elevated levels of angiogenic CAFs (angiogenic group and dual group) had unfavorable clinical outcomes compared to patients in the inflammatory group, but the difference was not statistically significant (median OS 11.2 vs. 16.7 months, p = 0.301; median PFS 6.0 vs. 5.1 months, p = 0.697; Supplementary Fig. 1).
Cluster analysis of cytokines and angiogenic factors (CAF). Unsupervised cluster analysis of CAFs in patients with gastric cancer. Dendrograms show 68 patient samples (columns) according to 42 soluble CAFs (rows). The CAF concentration ratios are depicted by a log-transformed pseudo-color intensity scale. Three patient groups were identified: angiogenic, inflammatory, and dual. Please see Table 2 for a complete list of CAFs
CAF profile according to pathological characteristics of GC
CAF analytes were compared according to HER2 status, Lauren classification, and signet ring cell components. GRO-α was significantly lower in patients with HER2-positive status than in patients with HER2-negative status (p = 0.037; Fig. 2a), and GRO-α was higher in patients with diffuse-type disease compared to patients with intestinal-type disease (p = 0.047; Fig. 2b). Also, sCA9 was lower in patients with diffuse-type disease than in patients with intestinal-type disease (p = 0.037; Fig. 2c). Stem cell factor (SCF) and macrophage inflammatory protein 1-alpha (MIP-1α) were lower in patients with signet ring cell components than in patients without signet ring cell components (p = 0.037 and p = 0.038, respectively; Fig. 2d, e). All CAF analytes are summarized in Supplementary Fig. 2 according to HER2 status, Lauren classification, and signet ring cell components.
Differences in CAF profiles according to HER2 status, Lauren type classification, and signet ring cell components. Mean levels (X on left side) plus 2 standard errors (bar) and all levels (dots on right side) of significantly different CAFs according to HER2 status (a), Lauren classification (b, c), and signet ring cell components (SRC, d, e). Relative levels of CAFs were calculated by dividing each value by the mean level of HER2-negative status (a), intestinal-type Lauren classification (b, c), and signet ring cell components (d, e), respectively. GRO-a growth-regulated alpha protein, MIP-1a macrophage inflammatory protein 1-alpha, sCA9 soluble carbonic anhydrase 9, SCF stem cell factor
CAF groups predict OS in GC
For each CAF, repeat analyses were performed to compare the OSs of patient groups divided by each level of CAF and identify the optimal cutoff points for predicting OS. A total of 42 CAFs were analyzed, and 11 CAFs significantly predicted OS: IL-2 receptor-alpha, GRO-α, HGF, M-CSF, SDF-1α, IL-6, IL-8, IL-10, interferon-gamma (IFN-γ), VEGF, and OPN (Table 3). We then classified patients into two groups by clustering analysis using these significant 11 CAFs. Clustering analysis of these 11 CAFs revealed two distinct groups: patients with high levels of these CAFs (high 11-CAF signature, N = 42) and patients with low levels of these CAFs (low 11-CAF signature, N = 26; Fig. 3a). The median OS of the high 11-CAF signature group was 10.1 months and the median OS of the low 11-CAF signature group was 17.9 months (HR 1.77, p = 0.026; Fig. 3b). The PFS of high 11-CAF signature group was significantly prolonged compared to that of low 11-CAF signature group (HR 2.04, p = 0.012; Fig. 3c). Interestingly, clinicopathological characteristics were not significantly different between the high and low 11-CAF signature groups (Supplementary Table 2). Moreover, multivariate Cox analysis showed that the high 11-CAF signature was independently correlated with poor OS along with poor performance status and the presence of signet ring cell components (HR 1.76, p = 0.029; Supplementary Table 3).
Cluster analysis of 11-CAF signature. Unsupervised cluster analysis of 11 CAFs that significantly predicted poor overall survival. Dendrograms show 68 patient samples (columns) with baseline levels of 11 significant CAFs (rows). The CAF concentration ratios are depicted by a log-transformed pseudo-color intensity scale. Two patient groups were identified: high 11-CAF signature and low 11-CAF signature (a). Kaplan–Meier curves for overall survival (b) and progression-free survival (c) according to the 2 groups identified by cluster analysis of the 11-CAF signature. CI confidential interval, HR hazard ratio, mOS median overall survival, IL-2Ra interleukin-2 receptor alpha, HGF hepatocyte growth factor, M-CSF macrophage colony-stimulating factor, SDF-1a stromal cell-derived factor 1 alpha, VEGF vascular endothelial growth factor, GRO-a growth-regulated alpha protein, SDF-1a stromal cell-derived factor 1-alpha, OPN osteopontin, IFN-γ interferon-gamma
OS prediction modeling with IL-8 and OPN
Eleven CAFs that significantly predicted OS were analyzed with other clinicopathological characteristics (Supplementary Table 4). Univariate Cox analysis of OS showed that performance status and signet ring cell components, along with 11 CAFs, were significantly correlated with poor OS. Multivariate Cox analysis showed that IL-8 (HR 2.31, p = 0.009) and OPN (HR 2.70, p = 0.001) were significantly correlated with poor OS. Using these 2 CAFs, which showed the strongest relationships with OS, we developed a model for predicting OS. The median OS of patients with elevated levels of both IL-8 and OPN was 8.2 months; the median OSs of patients with elevated levels of either one of these factors or neither factor were 15.8 and 19.9 months, respectively (p = 0.002 and p < 0.001, respectively; Supplementary Fig. 3a). The median PFS of patients with elevated levels of both IL-8 and OPN was worse than other groups (p = 0.068 and 0.007; Supplementary Fig. 3b).
Discussion
In this study, we analyzed pretreatment serum levels of 52 CAFs in patients with advanced GC. Clustering analysis of the CAFs revealed three distinct patient groups: patients with high levels of angiogenic factors, patients with high levels of inflammatory mediators, and patients with high levels of both angiogenic factors and inflammatory mediators. We found the 11 CAF-signature could identify patients who obtained poor prognosis when treated with standard conventional chemotherapy.
Although correlations between inflammation and cancer are well established [10, 22] and angiogenic sprouting of tumors is known as an important feature of carcinogenesis and cancer progression [23], few studies have been reported that define solid cancers according to circulating factors, such as CAFs. Clustering analyses of pretreatment serum CAFs to classify patients into subgroups have been reported in renal cell carcinoma and non-small cell lung cancer [16, 19], and several studies showed that a distinct CAF profile could predict clinical outcomes [15–19]. However, although associations between inflammatory cascades or angiogenic factors, such as VEGF, and clinical outcomes have been reported in GC [24], comprehensive explorations of circulating factors and the relationships with clinical outcomes have not been published for GC.
We investigated pretreatment serum levels of 52 CAFs in GC patients. To the best of our knowledge, this is the most extensive investigation of CAFs in GC. Clustering analysis revealed three distinct groups of CAFs. One group, which was classified as the angiogenic group in a previous report of renal cell carcinoma, included VEGF, HGF, GRO-α, M-CSF, and OPN. The other group, which was also used in the previous report [16], included various IL series, as well as tumor necrosis factor-alpha and IFN-γ, which are well-known mediators of inflammatory cascades [10]. The third group included both factors. Although OSs were not significantly different among patients in the three CAF groups, patients who had elevated levels of angiogenic CAFs tended to have unfavorable OS (Supplementary Fig. 1). Moreover, 9 of the 11 CAFs that predicted poor OS according to the Cox analysis were classified as angiogenic CAFs. To the best of our knowledge, this is the first report to assess the interactions between circulating CAFs via clustering analysis in GC patients and identify patient subgroups on the basis of CAFs.
CAF levels varied according to pathological classifications of GC, such as HER2 status, Lauren classification [21], and signet ring cell components. GRO-α was significantly higher in HER2-negative GC and diffuse-type GC compared with HER2-positive GC and intestinal-type GC, respectively. GRO-α attracts immune cells via the chemokine receptor CXCR2 [25, 26]. Previous reports showed that GRO-α expression in tumors is higher in diffuse-type disease than intestinal-type disease [27]. Previous reports showed sCA9, SCF, and MIP-1a are associated with a hypoxic signature [18, 28–30]. As intestinal-type GC is partially caused by Helicobacter pylori-associated metaplasia and mediated by hypoxia-induced angiogenesis [31, 32], as such, increased levels of sCA9 in intestinal-type disease compared to the diffuse-type disease would be reasonable. Moreover, as signet ring cell pathology is associated with diffuse-type disease [21, 33], it is expected that serum levels of SCF and MIP-1a would be lower in patients with signet ring cell components. However, these findings and the statistical comparison of the groups according to HER2 status and Lauren classification should be interpreted with caution, because only a small number of patients (N < 10) were included in our study.
Previous studies of CAFs have indicated that an understanding of the delicate networking of CAFs is necessary to correlate CAF levels to clinical outcomes, because the biological activity of circulating factors might be the result of the interactions of CAFs rather than the action of single CAFs [15–19]. A previous study in renal cell carcinoma identified 6 CAFs as significant predictive factors for the selection of a combination regimen of sorafenib and interferon-alpha compared to sorafenib alone [16]. In head and neck squamous cell carcinoma, CAFs that were defined as a hypoxia signature were closely clustered and their levels were higher in patients who experienced progression after induction chemotherapy than in those who did not experience disease progression [18]. In our study, we identified 11 CAFs that independently predicted poor OS among 52 CAFs. A cluster analysis that included these 11 CAFs divided patients by prognosis, even after adjustment for other traditional significant clinicopathological features. Interestingly, the characteristics of patients were not different according to the 11-CAF signature, even though the OS was significantly different (Supplementary Table 2, Supplementary Table 3). According to The Cancer Genome Atlas of stomach adenocarcinoma [3], genomically stable tumor, which would be correlated with signet ring cell component or diffuse type, would have a high inflammatory signature; however, some portions of other molecular subtypes such as Epstein–Barr virus-positive, chromosomal instability, and microsatellite-high might also contain high CAF signature as a result of CD274 (PD-L1) amplification and VEGF-α amplification, and abundant neo-antigen-promoting immunogenicity [34, 35], respectively. Therefore, tumors with high inflammatory markers or an 11-CAF signature would not be simply associated with the conventional clinicopathological factors. This finding indicates that using traditional clinicopathological prognostic factors limits the ability to identify patients who are likely to experience a poor prognosis after standard chemotherapy in GC. Using 11-CAF signature information, we can more accurately predict the prognosis of patients who receive standard chemotherapy, which fills a substantial gap in medical knowledge.
A multivariate Cox analysis of each of the 11 CAFs revealed that high levels of IL-8 and OPN independently predicted poor survival (Supplementary Table 4). IL-8 is a proinflammatory cytokine that attracts and activates neutrophilic granulocytes and it is associated with poor prognosis in many cancers [36]. Moreover, the close association of IL-8 with tumor angiogenesis has been reported [37, 38] and the dual inhibition of VEGF and IL-8 has been suggested as a potentially efficient strategy for treating cancer [39]. Previous studies have shown that serum levels of IL-8 are higher in patients with GC than in healthy control patients [40, 41], and serum IL-8 has been reported as a poor prognostic factor in a variety of malignant diseases, including melanoma, renal cell carcinoma, and hepatocellular carcinoma [42]. Our study adds the evidence of correlation between serum IL-8 and poor prognosis in GC for the first time.
The role of OPN in tumor progression has been reported to be extracellular remodeling to promote epithelial-mesenchymal transition and angiogenesis [43, 44]. Studies have shown that OPN could bind CD44, then activate the phosphoinositide 3-kinase/protein kinase B pathway, and upregulate VEGF [45–47]. Several studies showed that tumor expression of OPN was a poor prognostic factor in resectable cases of GC [48], and serum levels of OPN have also been represented as significant prognostic factors in many cancers, including GC [49–51]. However, our study gives the information on the prognostic significance of OPN in advanced GC patients who are treated with conventional standard chemotherapy for the first time.
The current study presents a new scope of GC classification on the basis of serum CAF clustering and survival analysis, but there are several limitations that should be considered. Although all patients who enrolled in the study were successfully followed for the survival analysis, the retrospective design of the study and relatively small number of patients limits the interpretation of the results. However, 52 different serum CAFs in our study that were concurrently analyzed with the survival of GC patients contributes to discriminating the types of circulating factors that influence clinical outcomes and recognizing the delicate networking of CAFs. The most valuable finding of the current study is the identification of the 11-CAF signature, which may be able to discern GC patients with a poor prognosis when treated with standard chemotherapy, which otherwise could not be identified. Although previous reports have shown that simple biomarkers of cancer-associated inflammation, such as high C-reactive protein or low albumin levels, would relate to poor prognosis of GC [52, 53], the involved mechanisms have not been known thoroughly. Our study suggests that this biological impact would be mediated by the 11-CAF signature. However, this gross shot of the CAF signature should be further clarified in terms of its exact networking and actions that result in the final clinical outcomes of patients. For patients with a high 11-CAF signature, treatment strategies targeting the factors included in this signature might be worthy to be investigated. For example, for the patient selection for ramucirumab, an anti-VEGFR2 antibody which has recently proved survival benefits in the second-line setting of GC [54], a single factor such as VEGF-α is not enough to identify the optimal patients. In that case, an 11-CAF signature might be a candidate to be evaluated. Another aspect is that patients with high CAF signature might benefit from the immunological agents because the high inflammatory signature such as the IFN-γ signature has been suggested to be related to a favorable clinical outcome of immune checkpoint inhibitors [55].
In conclusion, serum CAF profiling differentiated three subgroups of GC patients: angiogenic, inflammatory, and dual groups. A high 11-CAF signature could identify GC patients with a poor prognosis when treated with standard chemotherapy, who need urgent new treatment strategies.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature (Lond). 2014;513:202–9.
Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–84.
Dulak AM, Schumacher SE, van Lieshout J, Imamura Y, Fox C, Shim B, et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 2012;72:4383–93.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Kang YK, Rha SY, Tassone P, Barriuso J, Yu R, Szado T, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660–6.
Salgia R, Patel P, Bothos J, Yu W, Eppler S, Hegde P, et al. Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin Cancer Res. 2014;20:1666–75.
Chang J, Wang S, Zhang Z, Liu X, Wu Z, Geng R, et al. Multiple receptor tyrosine kinase activation attenuates therapeutic efficacy of the fibroblast growth factor receptor 2 inhibitor AZD4547 in FGFR2 amplified gastric cancer. Oncotarget. 2015;6:2009–22.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (Lond). 2008;454:436–44.
Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature (Lond). 2012;481:85–9.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (Lond). 2014;515:568–71.
Muro K, Bang Y-J, Shankaran V, Geva R, Catenacci DVT, Gupta S, et al. Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012. ASCO Meet Abstr. 2015;33:3.
Montero AJ, Diaz-Montero CM, Millikan RE, Liu J, Do KA, Hodges S, et al. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon-alpha: association of pretreatment serum levels with survival. Ann Oncol. 2009;20:1682–7.
Zurita AJ, Jonasch E, Wang X, Khajavi M, Yan S, Du DZ, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:46–52.
Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–37.
Byers LA, Holsinger FC, Kies MS, William WN, El-Naggar AK, Lee JJ, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010;9:1755–63.
Barrera L, Montes-Servin E, Barrera A, Ramirez-Tirado LA, Salinas-Parra F, Banales-Mendez JL, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol. 2015;26:428–35.
Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Coussens LM, Werb Z. Inflammation and cancer. Nature (Lond). 2002;420:860–7.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature (Lond). 2011;473:298–307.
Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Graves CA, et al. Prognostic significance of serum levels of vascular endothelial growth factor and insulin-like growth factor-1 in advanced gastric cancer patients treated with FOLFOX chemotherapy. Chemotherapy. 2012;58:426–34.
Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–73.
Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, et al. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–59.
Eck M, Schmausser B, Scheller K, Brandlein S, Muller-Hermelink HK. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol. 2003;134:508–15.
Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64.
Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–68.
Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou B, et al. Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF). Carcinogenesis (Oxf). 2008;29:1853–61.
Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60:3–21.
Jung JH, Im S, Jung ES, Kang CS. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci. 2013;10:1217–23.
Hass HG, Smith U, Jager C, Schaffer M, Wellhauber U, Hehr T, et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): single-center experience of 160 cases. Onkologie. 2011;34:682–6.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74.
Zarogoulidis P, Katsikogianni F, Tsiouda T, Sakkas A, Katsikogiannis N, Zarogoulidis K. Interleukin-8 and interleukin-17 for cancer. Cancer Invest. 2014;32:197–205.
Lattanzio L, Tonissi F, Torta I, Gianello L, Russi E, Milano G, et al. Role of IL-8 induced angiogenesis in uveal melanoma. Invest New Drugs. 2013;31:1107–14.
Desai S, Laskar S, Pandey BN. Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cell Signal. 2013;25:1780–91.
Gyanchandani R, Sano D, Ortega Alves MV, Klein JD, Knapick BA, Oh S, et al. Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol. 2013;49:761–70.
Macri A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, et al. Serum levels of interleukin 1-beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184–93.
Epplein M, Xiang YB, Cai Q, Peek RM Jr, Li H, Correa P, et al. Circulating cytokines and gastric cancer risk. Cancer Causes Control. 2013;24:2245–50.
Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20:5697–707.
Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61.
Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem. 2003;278:28593–606.
Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, et al. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60–7.
Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: implications for cancer. Biochim Biophys Acta. 2015;1855:202–22.
Higashiyama M, Ito T, Tanaka E, Shimada Y. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007;14:3419–27.
Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–9.
Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552:61–85.
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–81.
Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489.
Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–41.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35.
Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21:3969–76.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant no. 2013R1A1A2008705) and supported by a grant from Seoul National University College of Medicine (800-20140609).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ock, CY., Nam, AR., Bang, JH. et al. Signature of cytokines and angiogenic factors (CAFs) defines a clinically distinct subgroup of gastric cancer. Gastric Cancer 20, 164–174 (2017). https://doi.org/10.1007/s10120-015-0583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0583-z