Abstract
Background
Laparoscopic distal gastrectomy (LDG) is an established procedure for the treatment of early gastric cancer. Roux-en-Y (R-Y) or Billroth-I (B-I) reconstruction is generally performed after LDG in Japan. The aim of this retrospective cohort study was to compare the effectiveness of R-Y and B-I reconstructions and thereby determine which has better clinical outcomes.
Methods
We analyzed data from 172 patients with gastric cancer who underwent LDG. Reconstruction was done by R-Y in 83 patients and B-I in 89. All patients were followed up for 5 years. Evaluated variables included symptoms, nutritional status, endoscopic findings, gallstone formation, and later gastrointestinal complications.
Results
Scores for the amount of residue in the gastric stump, remnant gastritis, and bile reflux, calculated according to the “residue, gastritis, bile” scoring system, were significantly lower in the R-Y group (score 0 vs. 1 and more; p = 0.027, <0.001, and <0.001, respectively). The proportion of patients with reflux esophagitis was significantly lower in the R-Y group (p < 0.001). Relative values (postoperative 5 years/preoperative) for body weight, serum albumin level, and total cholesterol level were similar in the two groups (p = 0.59, 0.56, and 0.34, respectively). Gallstone formation did not differ between the groups (p = 0.57). As for later complications, the incidence of gastrointestinal ulcer was 4.5 % in the B-I group, and that of ileus was 3.6 % in the R-Y group, but differences between the groups were not significant (p = 0.12, 0.11, respectively).
Conclusions
As compared with B-I, R-Y was associated with lower long-term incidences of both bile reflux into the gastric remnant and reflux esophagitis.
Similar content being viewed by others
Introduction
Laparoscopic distal gastrectomy (LDG) is now an established minimally invasive procedure for the treatment of gastric cancer. Two clinical studies have reported on the safety of LDG in patients with early-stage gastric cancer [1, 2]. As compared with open distal gastrectomy (ODG), LDG has several important advantages, including less intraoperative blood loss, less postoperative pain, earlier postoperative recovery, and shorter hospital stay [3–7]. Billroth-I (B-I), Billroth-II (B-II), and Roux-en-Y (R-Y) procedures have been used for reconstruction after both LDG and ODG. However, after gastrectomy some patients have disorders such as malabsorption, dumping syndrome, reflux esophagitis, alkaline gastritis, and delayed gastric emptying [8–10]. Duodenogastric reflux has been recognized to be a major cause of clinical symptoms after gastrectomy [11]. Several clinical and experimental studies have shown that reflux of bile acids and pancreatic proteolytic enzymes can damage the gastric mucosa and promote the development of gastritis, gastric ulcer, reflux esophagitis, and esophageal and gastric cancer [12–14]. Previous studies have compared postoperative complications and disorders between B-I and R-Y after distal gastrectomy, and we reported that R-Y reconstruction had better short-term outcomes than B-I reconstruction [15]. However, long-term studies of disorders after LDG are scant. The aim of this retrospective cohort study was to compare long-term clinical results between B-I and R-Y to determine which procedure is better.
Methods
At least 5 years had elapsed since the date of radical LDG with lymphadenectomy in a total of 222 patients with a histopathologically confirmed diagnosis of gastric adenocarcinoma who underwent surgery in our hospital between January 1999 and August 2006. The following patients were excluded: 19 who underwent B-II reconstruction, 21 who died (including 10 who died of disease recurrence), 2 who were treated for recurrent disease, 1 who underwent pancreaticoduodenostomy for cancer of the ampulla of Vater, and 7 who were lost to follow up. Clinical data for up to 5 years after the operation were available for 172 patients, 89 who underwent B-I reconstruction and 83 who underwent R-Y reconstruction.
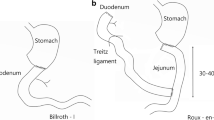
The procedure for LDG has been described previously [15]. The extent of lymph node dissection was decided according to tumor stage, classified according to the 13th version of the Japanese Gastric Cancer Classification [16] and the Treatment Guidelines of the Japanese Gastric Cancer Association [17]. The hepatic branches of the vagus nerve were preserved in all patients; the decision on whether to preserve the celiac branches of the vagus nerve was left up to the operator. Patients who underwent B-I reconstruction received a side-to-end gastroduodenostomy, generally performed extracorporeally with a circular stapler. R-Y reconstruction was performed with an isoperistaltic Roux limb (length 20–30 cm) divided 20–30 cm from the duodeno-jejunal flexure. Side-to-side gastrojejunostomy and side-to-side jejunojejunostomy were performed extracorporeally with a linear stapler. The Roux limb was brought up through the antecolic route. Between 1999 and 2003, B-I reconstruction was the main method used, and R-Y reconstruction was the main method used from 2004, when we modified the method for anastomosis.
Postoperative follow up included clinical, laboratory, and imaging (ultrasonography or computed tomography [CT]) examinations, performed every 6–12 months in patients with stage I disease and every 3–6 months in those with stage II or more advanced disease. After the operation, endoscopy was performed annually in most patients, but at 1, 3, and 5 years in some. Clinical symptoms, nutritional parameters, and the results of endoscopic examinations were compared between the R-Y group and B-I group 5 years after the operation. Clinical symptoms such as gastroesophageal reflux (e.g., heartburn, chest pain, regurgitation, and nausea) and dumping syndrome (e.g., palpitations, hot flush, perspiration, vertigo, abdominal pain, and hypoglycemia) were assessed by personal interviews with the patients. We confirmed whether antacid drugs were prescribed to treat gastroesophageal reflux. Endoscopic examinations were performed to evaluate the lower esophageal mucosa and gastric remnant stump and to assess the presence of bile and the amount of residue in the gastric stump. Endoscopic findings of the gastric remnant were evaluated according to the “residue, gastritis, bile” (RGB) classification system [18]. Signs and symptoms of gastroesophageal reflux disease were evaluated according to the modified Los Angeles classification system [19]. Body weight, serum albumin level, and serum total cholesterol level were measured as nutritional parameters, and the relative values (ratio of the postoperative value at 5 years to the preoperative value) were calculated. Abdominal ultrasonography or CT was performed to evaluate concurrent conditions such as gallstone formation and postoperative recurrence. In addition, we compared later gastrointestinal complications between the R-Y and B-I groups. “Later complications” in this study were defined as those that developed more than 1 month after the operation.
Statistical analysis
Categorical data were compared with the use of the χ2 test, Yates’ correction, or Fisher’s exact test, as appropriate. Other variables, such as serum albumin level and serum total cholesterol level, were compared with the use of the Mann–Whitney U-test. Values of p < 0.05 were considered to indicate statistical significance. All analyses were performed with the statistical software package SPSS 17 (SPSS Japan, Tokyo, Japan).
Results
The characteristics of the patients are shown in Table 1. Age, gender, and stage of gastric cancer were similar in the R-Y group and B-I group. The proportion of patients who underwent D1 + α lymph node dissection was significantly lower and body mass index was significantly higher in the R-Y group (p = 0.010, p = 0.036, respectively). The rate of vagus nerve preservation (both the hepatic and celiac branches) was significantly lower in the R-Y group (59 %) than in the B-I group (84 %; p < 0.001).
Clinical symptoms were assessed in all patients. Four patients (5 %) in the R-Y group and 10 (11 %) in the B-I group had symptoms of gastroesophageal reflux (p = 0.17). Dumping syndrome was diagnosed in 4 patients (5 %) in the R-Y group and 9 (10 %) in the B-I group (p = 0.25). The differences in the incidences of these symptoms did not differ significantly between the groups (Table 2).
Endoscopy findings were available for 159 patients (92 %). The RGB scores for the amount of residue (score 0 vs. 1 and more, p = 0.027), remnant gastritis (p < 0.001), and bile reflux into the remnant stomach (p < 0.001) were significantly lower in the R-Y group than in the B-I group (Table 3). No patient in the R-Y group had reflux esophagitis of grade A or greater severity according to the modified LA classification. The proportion of patients with grade M or more severe reflux esophagitis was significantly lower in the R-Y group (34 vs. 70 %, p < 0.001) (Table 4). Body weight data were available for 161 patients (94 %). Relative body weight did not differ between the R-Y and the B-I groups (p = 0.59). Laboratory data were available for 162 patients (94 %). Relative values of serum albumin and total cholesterol were similar in the two groups (p = 0.56, p = 0.34, respectively) (Table 5).
Gallstones developed in 12 patients (17 %) in the R-Y group and 10 (12 %) in the B-I group, excluding patients who had had undergone cholecystectomy; this difference was not significant (p = 0.57). The incidence of gallstones also did not differ according to whether the celiac branch of the vagus nerve was preserved or removed (p = 0.46). There was also no difference in the incidence of gallstones according to the extent of lymph node dissection (p = 0.77) (Table 6).
Later gastrointestinal complications occurred in 4 patients (4.8 %) after R-Y and 6 (6.7 %) after B-I; this difference was not significant (p = 0.75). Gastrointestinal ulcers developed in 4 patients (4.5 %) in the B-I group, but not in any patients in the R-Y group. Ileus occurred in 3 patients (3.6 %) in the R-Y group, but not in any patients in the B-I group. One patient (1.2 %) in the R-Y group had bleeding from the jejunojejunostomy. One patient (1.1 %) in the B-I group who had undergone cholecystectomy had cholangitis without common bile duct stones. There were no significant differences in any complications between the two groups (Table 7).
Discussion
Our study suggests that the functional advantages of R-Y can continue for a prolonged period after LDG. Evaluation of long-term clinical outcomes demonstrated that patients who underwent R-Y reconstruction had less residue in, and less bile reflux into, the remnant stomach. Consequently, the incidence of gastritis or esophagitis on endoscopic examination was lower in the R-Y group than in the B-I group. We previously evaluated short-term outcomes after LDG and reported several advantages of R-Y over B-I, consistent with the results of the present study [15]. Previous studies of short-term outcomes have shown that R-Y reconstruction, compared with B-I, is associated with lower incidences of gastroesophageal reflux, bile reflux, and remnant gastritis on endoscopic examination [12, 20–22]. Gastroesophageal reflux, as assessed by dynamic scintiscan, is also less frequent after R-Y than after B-I [23]. In addition, bile reflux on bilirubin monitoring and biliary scintigraphy is mild after R-Y [12, 21]. Less gastroesophageal and duodenogastric reflux might underlie the lower incidence of symptoms after R-Y. In other studies [12, 21], the proportions of patients with clinical symptoms caused by these types of reflux ranged from 0 to 17 % after R-Y, as compared with 12–63 % after B-I 3–6 months after operation. The incidence of heartburn was lower after R-Y (10 %) than after B-I (37 %) 1 year postoperatively in our previous study [15]. However, the incidence of reflux symptoms in the present study (R-Y 5 %; B-I 11 %) was lower than that in our previous study, although 70 % of the patients were the same. Another large survey of R-Y and B-I, after ODG, reported lower incidences of heartburn (0 and 6.4 %, respectively) and regurgitation (2.5 and 1.5 %, respectively) with R-Y at 5 years [24]. Medical therapy or improved diet may reduce reflux symptoms for several years after distal gastrectomy. Food residue was significantly less in the R-Y group in the present study, suggesting that R-Y may be associated with less discomfort after meals. However, we did not use internationally validated questionnaires, such as that recommended by the European Organisation for Research and Treatment of Cancer, to assess clinical symptoms [25]. Inadequate assessment of patients’ symptoms was thus a limitation of our study. Further detailed questionnaire surveys are needed to establish whether R-Y decreases clinical symptoms. We assume that questionnaires such as the “frequency scale for the symptoms of gastroesophageal reflux disease” (FSSG) may be useful for evaluating the benefits of R-Y, which reduces reflux esophagitis [26].
Nunobe et al. demonstrated that R-Y significantly increased gallstone formation as compared with B-I during long-term follow up after ODG (28.0 vs. 15.3 %, p = 0.005) [24]. On the other hand, Fukagawa et al. reported similar incidences of gallstone formation after R-Y (26.9 %) and B-I (24.0 %). Moreover, a greater extent of lymph node dissection was associated with a higher incidence of gallstones [27]. Similar results were obtained in another study including patients who underwent open gastrectomy (D1 9.4 %; D2 17.8 %) [28]. Apart from the passage of food through the duodenum, destruction of the vagus nerve is also an important risk factor for gallstone formation [29] and such destruction was marked in patients who had undergone extensive lymph node dissection. In a previous study of long-term outcomes, including some patients who underwent pylorus-preserving gastrectomy [30], the incidence of gallstones after ODG was substantially reduced (1.8 %) by preserving the vagus nerve. In the present study, the rate of gallstone formation was 14 %, although the hepatic branch of the vagus nerve was preserved in all patients and the celiac branch was preserved in most. In addition, D2 dissection did not increase the risk of gallstone formation. This apparent discrepancy may be explained by the following factors. Gallbladder function is regulated not only by the hepatic branches of the vagus nerve, but also by the retroperitoneal sympathetic and parasympathetic nerves [29]. When D1 + α or D1 + β lymph node dissection was performed in the present study, part of the nerve plexus along the common hepatic artery or the celiac artery might have been damaged by complete dissection of the No. 8a or No. 9 lymph nodes. Furthermore, ultrasonically activated devices were mainly used to perform lymph node dissection, and heat generated at the time of dissection might have injured the preserved nerves. No patient in the present study had symptomatic cholecystolithiasis or received treatment for gallstones. In contrast, a previous study reported that cholecystolithiasis developed in 27 % of patients with gallstones after open gastrectomy, and 46 % of these patients received surgical treatment [28].
The reconstruction method of choice after LDG remains controversial. B-I and R-Y procedures are the most widely used. Some reasons for favoring B-I may be its technical ease and the maintenance of digestive-system-related homeostasis and food flow into the duodenum. However, the passage of food through the duodenum may not be an important determinant of nutritional status after distal gastrectomy. Nutritional indexes such as serum albumin levels, serum total cholesterol levels, and body weight are similar after R-Y and B-I in the short term [15, 20], as well as in the long term, as demonstrated by the present study. Consistent with the results of our previous study, these nutritional indexes were similar in the R-Y and B-I groups in the present study at 6 months, 1 year, and 5 years after the operation. The size of the remnant stomach was an important determinant of the quality of life after gastrectomy. In another study [31], a larger remnant stomach after distal gastrectomy was associated with benefits such as less reflux esophagitis, more stable body weight, and better food intake in the B-I group. In patients with cancer arising in the lower third of the stomach, a larger remnant stomach can improve nutritional status and reduce symptoms [31]. We did not accurately measure the size of the remnant stomach in our series, but the upper third of the stomach was preserved in most patients. The size of the remnant stomach was less than one half in all patients. In patients in whom tension on the gastrointestinal anastomosis was a concern, the remnant stomach was slightly larger than the upper-third portion. As for late gastrointestinal complications, ulcers developed only after B-I in the present study. Stomal ulcer is a concern in R-Y because of less alkaline bile reflux into the stomach, potentially leading to acid-induced jejunal injury [32]. However, to avoid excessive tension on the gastroduodenostomy, the size of the remnant stomach might be larger and, consequently, acid production might be higher in B-I. On the other hand, the incidence of ileus was higher in the R-Y group in our study. Internal hernia after R-Y has been reported in patients who underwent gastric bypass surgery or LDG [33, 34]. One patient (1.2 %) in our R-Y group had an internal hernia, as compared with no patients in the B-I group. Mesenteric defects, including Petersen’s defect, should be closed in LDG, as is done in R-Y gastric bypass surgery [34]. Roux stasis syndrome is characterized by symptoms of upper gut stasis after R-Y gastrojejunostomy and is thought to be caused by an ectopic pacemaker that arises in the proximal part of the Roux limb divided from the natural small bowel pacemaker [35]. However, Roux stasis syndrome was not diagnosed in any of our patients for 5 years after the operation, although two patients (2.4 %) had disturbed food passage associated with an expanding remnant stomach and a bent Roux limb during the early postoperative course. Roux stasis syndrome has been attributed to removal of the vagus nerves and the length of the Roux-Y limb [36, 37]. We decided to use a Roux limb less than 30 cm in length on the basis of the results of previous studies [37]. Endoscopy for the papilla of Vater, or endoscopic retrograde cholangiopancreatography was impossible in R-Y. However, balloon enteroscopy is useful for these examinations [38]. Remnant gastric cancer (RGC) is also an important issue after gastrectomy. Patients in whom reconstruction is performed with B-II procedures at the first operation have the highest risk of RGC. The development of RGC is attributed primarily to duodenogastric reflux and hypochlorhydria [39]. R-Y may decrease the risk of secondary gastric cancer, but further long-term follow up is necessary to reach a definite conclusion on this risk. Low incidences of RGC have been reported after R-Y reconstruction, because B-I or B-II has been the main method employed [39, 40].
In conclusion, as compared with B-I, R-Y was associated with lower long-term incidences of both bile reflux into the gastric remnant and reflux esophagitis. Late complications and nutritional status did not differ between the R-Y and B-I groups.
References
Lee JH, Kim YW, Ryu KW, Lee JR, Kim CG, Choi IJ, et al. A phase-II clinical trial of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer patients. Ann Surg Oncol. 2007;14:3148–53.
Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238–44.
Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short term outcomes after laparoscopic-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–83.
Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–73.
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10.
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–11.
Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145–9.
Wu CW, Hsieh MC, Lo SS, Lui WY, P’Eng FK. Quality of life of patients with gastric adenocarcinoma after curative gastrectomy. World J Surg. 1997;21:777–82.
Tomita R, Tanjoh K, Fujisaki S, Fukuzawa M. Relation between gastroduodenal interdigestive migrating motor complex and postoperative gastrointestinal symptoms before and after cisapride therapy following distal gastrectomy for early gastric cancer. World J Surg. 2000;24:1250–7.
Woodward ER, Hocking MP. Postgastrectomy syndromes. Surg Clin North Am. 1987;67:509–20.
Svensson JO. Duodenogastric reflux after gastric surgery. Scand J Gastroenterol. 1983;18:729–34.
Osugi H, Fukuhara K, Takada N, Takemura M, Kinoshita H. Reconstructive procedure after distal gastrectomy to prevent remnant gastritis. Hepatogastroenterology. 2004;51:1215–8.
Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, et al. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery. 1997;122:874–81.
Sato T, Miwa K, Sahara H, Segawa M, Hattori T. The sequential model of Barrett’s esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens. Anticancer Res. 2002;22:39–44.
Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg. 2008;247:962–7.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 13 ed. Tokyo: Kanehara; 1999 (in japanese)
Japanese Gastric Cancer Association. Gastric cancer treatment guidelines for doctors' reference. Tokyo: Kanehara; 2001 (in Japanese)
Nagano H, Ohyama S, Sakamoto Y, Ohta K, Yamaguchi T, Muto T, et al. The endoscopic evaluation of gastritis, gastric remnant residue, and the incidence of secondary cancer after pylorus-preserving and transverse gastrectomies. Gastric Cancer. 2004;7:54–9.
Miwa H, Yokoyama T, Hori K, Sakagami T, Oshima T, Tomita T, et al. Interobserver agreement in endoscopic evaluation of reflux esophagitis using a modified Los Angeles classification incorporating grades N and M: a validation study in a cohort of Japanese endoscopists. Dis Esophagus. 2008;21:355–63.
Ishikawa M, Kitayama J, Kaizaki S, Nakayama H, Ishigami H, Fujii S, et al. Prospective randomized trial comparing Billroth I and Roux-en-Y procedures after distal gastrectomy for gastric carcinoma. World J Surg. 2005;29:1415–20.
Shinoto K, Ochiai T, Suzuki T, Okazumi S, Ozaki M. Effectiveness of Roux-en-Y reconstruction after distal gastrectomy based on an assessment of biliary kinetics. Surg Today. 2003;33:169–77.
Tanaka S, Matsuo K, Matsumoto H, Maki T, Nakano M, Sasaki T, et al. Clinical outcomes of Roux-en-Y and Billroth I reconstruction after a distal gastrectomy for gastric cancer: what is the optimal reconstructive procedure? Hepatogastroenterology. 2011;58:257–62.
Montesani C, D’Amato A, Santella S, Pronio A, Giovannini C, Cristaldi M, et al. Billroth I versus Billroth II versus Roux-en-Y after subtotal gastrectomy. Prospective [correction of prespective] randomized study. Hepatogastroenterology. 2002;49:1469–73.
Nunobe S, Okaro A, Sasako M, Saka M, Fukagawa T, Katai H, et al. Billroth 1 versus Roux-en-Y reconstructions: a quality-of-life survey at 5 years. Int J Clin Oncol. 2007;12:433–9.
Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357–64.
Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–91.
Fukagawa T, Katai H, Saka M, Morita S, Sano T, Sasako M. Gallstone formation after gastric cancer surgery. J Gastrointest Surg. 2009;13:886–9.
Akatsu T, Yoshida M, Kubota T, Shimazu M, Ueda M, Otani Y, et al. Gallstone disease after extended (D2) lymph node dissection for gastric cancer. World J Surg. 2005;29:182–6.
Rehnberg O, Haglund U. Gallstone disease following antrectomy and gastroduodenostomy with or without vagotomy. Ann Surg. 1985;201:315–8.
Ando S, Tsuji H. Surgical technique of vagus nerve-preserving gastrectomy with D2 lymphadenectomy for gastric cancer. ANZ J Surg. 2008;78:172–6.
Nomura E, Lee SW, Bouras G, Tokuhara T, Hayashi M, Hiramatsu M, et al. Functional outcomes according to the size of the gastric remnant and type of reconstruction following laparoscopic distal gastrectomy for gastric cancer. Gastric Cancer. 2011;14:279–84.
Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647–51.
Kumagai K, Hiki N, Nunobe S, Jiang X, Kubota T, Aikou S, et al. Different features of complications with Billroth-I and Roux-en-Y reconstruction after laparoscopy-assisted distal gastrectomy. J Gastrointest Surg. 2011;15:2145–52.
Steele KE, Prokopowicz GP, Magnuson T, Lidor A, Schweitzer M. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg Endosc. 2008;22:2056–61.
Morrison P, Miedema BW, Kohler L, Kelly KA. Electrical dysrhythmias in the Roux jejunal limb: cause and treatment. Am J Surg. 1990;160:252–6.
van der Mijle HC, Beekhuis H, Bleichrodt RP, Kleibeuker JH. Transit disorders of the gastric remnant and Roux limb after Roux-en-Y gastrojejunostomy: relation to symptomatology and vagotomy. Br J Surg. 1993;80:60–4.
Gustavsson S, Ilstrup DM, Morrison P, Kelly KA. Roux-Y stasis syndrome after gastrectomy. Am J Surg. 1988;155:490–4.
Moreels TG, Pelckmans PA. Comparison between double-balloon and single-balloon enteroscopy in therapeutic ERC after Roux-en-Y entero-enteric anastomosis. World J Gastrointest Endosc. 2010;2:314–7.
Ahn HS, Kim JW, Yoo MW, Park do J, Lee HJ, Lee KU, et al. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632–9.
Takeno S, Noguchi T, Kimura Y, Fujiwara S, Kubo N, Kawahara K. Early and late gastric cancer arising in the remnant stomach after distal gastrectomy. Eur J Surg Oncol. 2006;32:1191–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inokuchi, M., Kojima, K., Yamada, H. et al. Long-term outcomes of Roux-en-Y and Billroth-I reconstruction after laparoscopic distal gastrectomy. Gastric Cancer 16, 67–73 (2013). https://doi.org/10.1007/s10120-012-0154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0154-5