Abstract
Background
It is unclear whether S-1 plus cisplatin is effective for patients with recurrent gastric cancer after adjuvant S-1 chemotherapy.
Methods
We retrospectively evaluated the efficacy of S-1 plus cisplatin in patients whose gastric cancer recurred after adjuvant S-1 chemotherapy.
Results
In the 52 patients evaluated, the median duration of adjuvant S-1 chemotherapy was 8.1 months, and the median recurrence-free interval (RFI) since the last administration of adjuvant S-1 was 6.4 months. Among the 36 patients with measurable lesions, 7 achieved a complete or partial response, and 13 were evaluated as having stable disease, for an overall response rate of 19.4% and a disease control rate of 55.6%. For all patients, the median progression-free survival (PFS) was 4.8 months, and the median overall survival (OS) was 12.2 months. Compared with patients with an RFI of <6 months (n = 25), patients with an RFI of ≥6 months (n = 27) had a significantly higher response rate (5.0 vs. 37.5%, respectively), longer PFS (2.3 vs. 6.2 months, respectively), and longer overall survival (7.3 vs. 16.6 months, respectively). According to a multivariate Cox model including performance status (PS) and reason for discontinuation of adjuvant S-1, an RFI of 6 months was still significantly associated with PFS and OS.
Conclusions
S-1 plus cisplatin is effective for patients with gastric cancer that recurs after adjuvant S-1 chemotherapy, especially for those with an RFI of ≥6 months.
Similar content being viewed by others
Introduction
Gastric cancer is the fourth most common malignancy in the world (988,602 cases in 2008, 7.8% of total malignancy cases) and the second leading cause of cancer death (737,419 deaths, 9.7% of total) [1]. The prognosis of patients with advanced or recurrent gastric cancer remains poor; chemotherapy confers only a minimal survival advantage, with a median survival of approximately 1 year. The most commonly used regimens are combination chemotherapy consisting of a fluoropyrimidine [5-fluorouracil (5-FU) or oral fluoropyrimidine] plus a platinum agent with or without docetaxel or anthracyclines [2–6].
S-1 is an oral anticancer drug composed of the 5-FU prodrug tegafur and two 5-FU modulators; it has achieved high response rates in patients with gastric cancer in phase II studies [7, 8]. In the Japan Clinical Oncology Group (JCOG) 9912 trial, which compared S-1, cisplatin plus irinotecan, and 5-FU, S-1 demonstrated non-inferiority compared to 5-FU [9]. In another phase III trial that compared S-1 alone to S-1 plus cisplatin (SPIRITS trial), S-1 plus cisplatin showed a significantly higher response rate (54 vs. 31%), longer progression-free survival (PFS; 6.0 vs. 4.0 months), and longer overall survival (OS; 13 vs. 11 months) [4]. Also, in a large, non-Japanese, phase III trial (the First-Line Advanced Gastric Cancer Study; FLAGS trial), S-1 plus cisplatin was associated with fewer toxic effects and demonstrated non-inferiority compared with 5-FU plus cisplatin by exploratory analysis [6]. Therefore, S-1 plus cisplatin is now considered to be one of the standard regimens for metastatic or recurrent gastric cancer.
In addition, the ACTS-GC trial has demonstrated that S-1 is also effective as adjuvant chemotherapy for Japanese patients who have undergone curative gastrectomy for locally advanced gastric cancer [10]. However, approximately 30% of patients still develop recurrence after curative resection followed by adjuvant S-1 [10]. As few patients who received adjuvant chemotherapy were included in the phase III trials described above [4, 7, 9], it is unclear whether patients who develop recurrence after adjuvant S-1 could achieve efficacy with S-1 plus cisplatin similar to that achieved in patients without adjuvant chemotherapy. To address this issue, we conducted the following multi-institutional retrospective analysis.
Patients and methods
Patients
This retrospective study was designed to evaluate the efficacy of first-line chemotherapy with S-1 plus cisplatin for recurrence in patients with gastric cancer who had undergone curative gastrectomy followed by adjuvant S-1 chemotherapy. Patients with histopathologically proven recurrent gastric adenocarcinoma after gastrectomy and lymph node dissection with no residual tumor were eligible for analysis. Additional eligibility criteria were: (1) previous adjuvant S-1 chemotherapy at a planned standard dose and schedule (80 mg/m2 for 28 consecutive days followed by a 14-day rest; 42-day cycles to be repeated for 1 year); (2) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; (3) adequate bone marrow, hepatic, and renal function to be treated with S-1 plus cisplatin; (4) evaluable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.1); and (5) treated with a standard regimen of S-1 plus cisplatin (S-1 80 mg/m2 for 21 consecutive days followed by a 14-day rest; cisplatin 60 mg/m2 intravenous infusion on day 8; 35-day cycles to be repeated) [4]. Written informed consent for treatment was obtained from each patient prior to treatment initiation. The Institutional Review Board of each participating center approved the study.
Evaluation of treatment and statistical analysis
The tumor response was assessed objectively according to RECIST ver. 1.1, and the best overall response was recorded as the antitumor effect for that patient. The disease control rate (DCR) represented the percentage of patients with a complete response (CR), partial response (PR), or stable disease (SD). PFS was measured from the date of initiation of S-1 plus cisplatin to the date of progressive disease or death from any cause. Time to treatment failure (TTF) was measured from the date of initiation of S-1 plus cisplatin to the date of last administration of S-1. OS was estimated from the date of initiation of S-1 plus cisplatin to the date of death or last follow-up visit, using the Kaplan–Meier method. The interval from the last administration of adjuvant S-1 to recurrence was defined as the recurrence-free interval (RFI).
The Cox proportional hazards model was used to estimate the impact of the RFI on TTF, PFS, and OS, with adjustment for other factors that were shown to be significant with a univariate log-rank test. P values for testing differences between proportions and response rates were calculated with χ2 tests for homogeneity or for trend, or with Fisher’s exact test. Results were considered to be statistically significant when the P value was <0.05. All reported P values are two-sided. In particular, we compared the response rate, DCR, time to progression (TTP), PFS, and OS between patients with RFIs of ≥6 and <6 months, because several clinical trials in the first-line setting set this interval of ≥6 months as an inclusion criterion [5, 9, 11].
Results
Patient characteristics
A total of 406 patients with recurrent gastric cancer after adjuvant S-1 chemotherapy had received chemotherapy at 18 institutions until October 2010. Among them, 57 patients (14.0%) had received S-1 plus cisplatin as first-line chemotherapy for recurrence. After the exclusion of 5 patients (1 patient with a non-evaluable lesion and 4 patients with insufficient data), 52 patients were included in the final analysis (Table 1). The median duration of adjuvant S-1 chemotherapy was 8.1 months (range 0.7–37.4 months), and the median RFI since the last administration of adjuvant S-1 was 6.4 months (range 0–81.3 months). Thirty of the 52 patients (57.7%) completed the planned duration of adjuvant S-1 therapy. In contrast, 14 patients discontinued S-1 due to disease recurrence, and 8 patients stopped therapy due to toxicity or patient refusal. Other than PS and liver metastasis, characteristics did not differ significantly between patients with an RFI of ≥6 months (n = 27) and those with an RFI of <6 months (n = 25) (Table 1).
Treatment results and efficacy
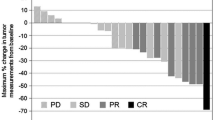
The median TTF was 4.1 months (95% confidence interval [CI] 2.5–5.1 months), with a median duration of follow-up of 32 months. Forty-four patients discontinued S-1 plus cisplatin due to disease progression (n = 40, 90.9%) or toxicity (n = 4, 9.1%). Of the 36 patients with measurable lesions, 7 achieved a CR (n = 3) or a PR (n = 4), and 13 were evaluated as having SD, for an overall response rate of 19.4% (95% CI 7.0–37.0%) and a DCR of 55.6% (95% CI 38.1–72.1%). The median PFS was 4.8 months (95% CI 3.9–6.2 months), and the median OS of all patients was 12.2 months (95% CI 10.2–16.6 months) (Fig. 1). Of the 44 patients who had discontinued S-1 plus cisplatin, 31 (70.4%) received second-line or third-line chemotherapy, including taxanes (n = 25) or irinotecan (n = 17).
Significance of the RFI
The response rate was significantly better in patients with an RFI of ≥6 months (37.5%; 95% CI 14–61%) than that in patients with an RFI of <6 months (5.0%; 95% CI 0–15%, P = 0.014, Table 2). In addition, compared with patients with an RFI of <6 months, patients with an RFI of ≥6 months had a significantly longer TTF (2.5 vs. 5.1 months, respectively, P = 0.025), longer PFS (2.3 vs. 6.2 months, respectively, P < 0.001, Fig. 2), and longer OS (7.3 vs. 16.6 months, respectively, P = 0.003, Fig. 2). According to a multivariate Cox model including PS and reason for discontinuation of adjuvant S-1, an RFI of 6 months was still significantly associated with PFS (hazard ratio [HR] 0.35, 95% CI 0.16–0.77, P = 0.009) and OS (HR 0.21, 95% CI 0.08–0.54, P = 0.001), although the association with TTF was not significant (HR 0.55, 95% CI 0.27–1.12, P = 0.1). When we divided the patients into two groups based on an RFI of 12 months, no significant difference between the groups was found in response rate, TTP, PFS, or OS.
Progression-free survival (PFS) and overall survival (OS) according to the length of the recurrence-free interval (RFI). Patients with an RFI of ≥6 months had a significantly longer median PFS (6.2 vs. 2.3 months, P < 0.001) and OS (16.6 vs. 7.3 months, P = 0.003) than patients with an RFI of <6 months. RFI recurrence-free interval, PFS progression-free survival, OS overall survival
Discussion
In the ACTS-GC study, adjuvant S-1 chemotherapy significantly improved the survival of patients who had undergone curative gastrectomy for locally advanced gastric cancer [10]. On the other hand, several small studies have suggested that patients with recurrence after adjuvant S-1 were refractory to S-1-containing regimens or had a worse prognosis compared with that of patients without adjuvant chemotherapy [12–14]. Although these reports never precluded the use of adjuvant S-1 chemotherapy, they raised the issue of how to treat recurrent disease after adjuvant S-1.
In the present retrospective study, we evaluated the efficacy of S-1 plus cisplatin in patients whose gastric cancer recurred after adjuvant chemotherapy with S-1. The response rate of 19.4% and PFS of 4.8 months were relatively worse compared with those in the SPIRITS study [4]. However, our results also suggested that patients with an RFI of ≥6 months who received S-1 plus cisplatin had a significantly better response rate, longer PFS, and longer OS compared to patients with an RFI of <6 months. The efficacy of S-1 plus cisplatin for patients with an RFI of ≥6 months in this study was almost compatible with that of patients in the SPIRITS trial in terms of PFS and OS, although these results should be interpreted cautiously due to the heterogeneity of the characteristics of the patients in the two studies. Although no prospective study has evaluated any chemotherapy specifically for patients who have failed adjuvant S-1, Kang and colleagues [15] conducted a phase II study of capecitabine plus cisplatin for 32 patients with gastric cancer that recurred after adjuvant chemotherapy with doxifluridine or 5-FU-containing regimens. They reported a response rate of 28% and a median TTP of 5.8 months, and concluded that capecitabine plus cisplatin was effective as first-line treatment in patients with recurrent gastric cancer after fluoropyrimidine-based adjuvant chemotherapy. In their report, the response rates (21 vs. 39%, P = 0.427), TTF (8.3 vs. 5.4 months, P = 0.072), and OS (14.1 vs. 9.3 months, P = 0.075) tended to be better in patients with an RFI of >6 months (n = 13) than in patients with an RFI of ≤6 months (n = 19), although the differences did not reach statistical significance [15]. These results were also consistent with those of previous studies in patients with other types of cancer, which suggested the importance of the RFI or treatment-free interval as a predictive marker of responsiveness to similar types of chemotherapy after recurrence [16–18]. Additionally, in the present study, the RFI cut-off value of 6 months was better than that of 12 months for predicting better outcomes and this finding may support the use of the conventional exclusion criteria in clinical trials in the first-line setting, which excluded patients who experienced disease recurrence within 6 months after the last adjuvant chemotherapy [5, 9, 11]. Therefore, selected patients with an RFI of ≥6 months with sufficient organ function may be adequately treated as chemo-naïve patients with standard chemotherapies such as S-1 plus cisplatin.
In contrast to the results for patients with an RFI of ≥6 months, the response rate in patients with an RFI of <6 months in the present study seemed to be worse than that of commonly used second-line chemotherapy regimens such as irinotecan and taxane combinations, which have a reported response rate of approximately 20% for patients with gastric cancer who received prior chemotherapy with fluoropyrimidines alone [18–23]. Based on these results, it may be suggested that the evaluation of chemotherapy regimens other than S-1 plus cisplatin might be warranted for the initial treatment of gastric cancer recurrence after adjuvant S-1. The response rate of 5.0% in our subset of patients with an RFI of <6 months was also lower than that reported previously by Kang et al. for capecitabine plus cisplatin after adjuvant chemotherapy (21%) [15]. The exact reasons for this difference are unknown. One possible reason is that Kang and colleagues did not use the same fluoropyrimidine (capecitabine after doxifluridine or 5-FU), and this choice might have contributed to a higher response in regard to early recurrence, although rechallenge with different types of fluoropyrimidine after the failure of another drug is still controversial in several types of cancer [24–28]. Second, the planned dose intensity of cisplatin as another key drug for gastric cancer was higher in their capecitabine plus cisplatin regimen (60 mg/m2 every 3 weeks) [15] than that in the S-1 plus cisplatin regimen (60 mg/m2 every 5 weeks). The efficacy of capecitabine plus cisplatin compared with other chemotherapy (irinotecan, taxane or irinotecan plus cisplatin) for recurrence after adjuvant S-1 should be evaluated in future clinical trials.
It is important to note the limitations of the present study. First, it was retrospective, and treatment after recurrence was selected by each physician individually. Considering the low proportion of patients who received S-1 plus cisplatin after recurrence (14.0%), the selected population may have been biased toward patients with good performance status (PS) and low tumor burden. Second, toxicity was not evaluated in this study, although the proportion of patients who discontinued S-1 plus cisplatin due to toxicity was low. Third, human epidermal growth factor receptor 2 (HER2) status was not evaluated. Trastuzumab, a humanized monoclonal antibody against HER2, has recently been shown to improve the prognosis of HER2-positive advanced gastric cancer [29], and the HER2 status of all gastric cancer types should be evaluated, even in this setting of recurrent disease. Fourth, the moderate sample size in a single-country study is another limitation; therefore, it would be better to validate the significance of the RFI after adjuvant failure on the PFS in other cohorts as well.
In conclusion, this is the first report to have evaluated the efficacy of chemotherapy with S-1 plus cisplatin in patients with gastric cancer that recurred after adjuvant chemotherapy with S-1. S-1 plus cisplatin was effective in such patients, especially in those with an RFI of ≥6 months. Further well-defined, prospective trials in this important patient population are required to identify optimal treatment regimens.
References
International Agency for Research on Cancer. GLOBOCAN. http://www-dep.iarc.fr/CancerMondial.htm (2008). Accessed April 2011
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73.
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53.
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur–0.4 M gimestat–1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20.
Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–7.
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–9.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J Clin Oncol. 2011 Aug 15. [Epub ahead of print]
Shitara K, Muro K, Ura T, Takahari D, Yokota T, Sawaki A, et al. Chemotherapy for gastric cancer that recurs after adjuvant chemotherapy with S-1. Jpn J Clin Oncol. 2008;38:786–9.
Hasegawa H, Fujitani K, Kurokawa Y, Hirao M, Nakazuru S, Mita E, et al. Effect of S-1 adjuvant chemotherapy on survival following recurrence and efficacy of first-line treatment in recurrent gastric cancer. Chemotherapy. 2010;56:436–43.
Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, et al. Survival and prognosticators of gastric cancer that recurs after adjuvant chemotherapy with S-1. Gastric Cancer. 2011;14:150–4.
Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. Phase II study of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. Br J Cancer. 2005;92:246–51.
Pujade-Lauraine E, Paraiso D, Cure H, Germann N, Lortholary A, Lucas V, et al. Predicting the effectiveness of chemotherapy (Cx) in patients with recurrent ovarian cancer (ROC): a GINECO study. Proc Am Soc Clin Oncol 2002;21:abstract 829.
Takashima A, Shirao K, Hirashima Y, Takahari D, Okita N, Akatsuka S, et al. Chemosensitivity of patients with recurrent esophageal cancer receiving perioperative chemotherapy. Dis Esophagus. 2008;21:607–11.
de Gramont Lesparre AH, Chibaudel B, Bourges O, Perez-Staub N, Tournigand C, Maindrault-Goebel F, et al. Definition of oxaliplatin sensitivity in patients with advanced colorectal cancer previously treated with oxaliplatin-based therapy. J Clin Oncol. 2009;27:15s. (abstr 4024).
Futatsuki K, Wakui A, Nakao I, Sakata Y, Kambe M, Shimada Y, et al. Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. CPT-11 Gastrointestinal Cancer Study Group. Gan To Kagaku Ryoho. 1994;21:1033–8.
Taguchi T, Sakata Y, Kanamaru R, Kurihara M, Suminaga M, Ota J, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent gastric cancer: a Japanese Cooperative Study Group trial (group A). Gan To Kagaku Ryoho. 1998;25:1915–24.
Mai M, Sakata Y, Kanamaru R, Kurihara M, Suminaga M, Ota J, et al. A late phase II clinical study of RP56976 (docetaxel) in patients with advanced or recurrent gastric cancer: a Cooperative Study Group Trial (group B). Gan To Kagaku Ryoho. 1999;26:487–96.
Yamada Y, Shirao K, Ohtsu A, Boku N, Hyodo I, Saitoh H, et al. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol. 2001;12:1133–7.
Nakae S, Hirao M, Kishimoto T, Iijima S, Ishida H, Morimoto T, et al. Phase II study of bi-weekly CPT-11 + CDDP for patients with gastric cancer refractory to S-1 (OGSG 0504 study). J Clin Oncol 2008;26 (May 20 suppl; abstr 4571).
Lee JJ, Kim TM, Yu SJ, Kim DW, Joh YH, Oh DY, et al. Single-agent capecitabine in patients with metastatic colorectal cancer refractory to 5-fluorouracil/leucovorin chemotherapy. Jpn J Clin Oncol. 2004;34:400–4.
Yasui H, Yoshino T, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Retrospective analysis of S-1 monotherapy in patients with metastatic colorectal cancer after failure to fluoropyrimidine and irinotecan or to fluoropyrimidine, irinotecan and oxaliplatin. Jpn J Clin Oncol. 2009;39:315–20.
Takiuchi H, Goto M, Imamura H, Furukawa H, Imano M, Imamoto H, et al. Multi-center phase II study for combination therapy with paclitaxel/doxifluridine to treat advanced/recurrent gastric cancer showing resistance to S-1 (OGSG 0302). Jpn J Clin Oncol. 2008;38:176–81.
Ono A, Boku N, Onozawa Y, Hironaka S, Fukutomi A, Yasui H, et al. Activity of S-1 in advanced or recurrent gastric cancer patients after failure of prior chemotherapy, including irinotecan + cisplatin or fluorouracil (except S-1). Jpn J Clin Oncol. 2009;39:332–5.
Yamamoto D, Yoshida H, Iwase S, Odagiri H, Kitamura K. TS-1 in patients with capecitabine-resistant breast cancer. J Clin Oncol 27:15s, 2009 (suppl; abstr 1103).
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Acknowledgments
This work was supported by the Epidemiological and Clinical Research Information Network (ECRIN).
Conflict of interest
None of the authors have financial or personal conflicts of interest to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Shitara, K., Morita, S., Fujitani, K. et al. Combination chemotherapy with S-1 plus cisplatin for gastric cancer that recurs after adjuvant chemotherapy with S-1: multi-institutional retrospective analysis. Gastric Cancer 15, 245–251 (2012). https://doi.org/10.1007/s10120-011-0101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0101-x