Abstract
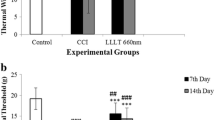
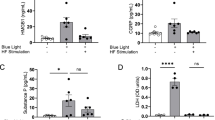
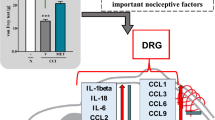
Nerve injury induces release of peptides and upregulation of receptors such as substance P and transient receptor potential receptor V1 (TRPV1), which contribute to the development and maintenance of chronic pain. Photobiomodulation therapy (PBMT) is a nonpharmacological strategy that promotes tissue repair and reduces pain and inflammation. However, the molecular basis for PBMT effects on neuropathic pain is still unclear. We investigated the effects of PBMT on substance P, TRPV1, and superficial temperature change in a rodent model of neuropathic pain. We evaluated substance P and TRPV1 in dorsal root ganglia (DRG L4 to L6) at baseline, 14 days after chronic constriction injury (CCI) and after PBMT. We also assessed the superficial temperature of tarsal, metatarsal, tibia, and fibula regions before and after PBMT using infrared thermography. Substance P and TRPV1 levels increased in DRG of CCI rats compared to naive and sham rats and decreased after PBMT. Infrared thermography showed increased temperature of tarsal, metatarsal, tibia, and fibula regions in CCI rats, which was decreased after PBMT. There were no statistical differences between CCI rats with PBMT, sham, and naive rats in any assay. PBMT reduces nociceptive mediators and hind paw and leg’s temperature in a rodent model of neuropathic pain, suggesting that PBMT may play a modulatory role in thermoregulation, neurogenic inflammation, and thermal sensitivity in peripheral nerve injuries. Therefore, PBMT appears to be a valuable strategy for neuropathic pain treatment in clinical settings.



Similar content being viewed by others
References
Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136(3):380–387
Ueda H (2006) Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther 109(1-2):57–77
Bennett GJ, Ochoa JL (1991) Thermographic observations on rats with experimental neuropathic pain. Pain 45(1):61–67
Brioschi ML, Myaki AE, Colman D, Rastelli MM Jr, Macedo RAC (2002) Infrared thermal imaging in rats submitted to different models of sciatic nerve and branches injuries. J Korean Med Thermology 2:64–65
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52(1):77–92
Cohen SP, Mao J (2014) Neuropathic pain: mechanisms and their clinical implications. Bmj 348:f7656. https://doi.org/10.1136/bmj.f7656
Malmberg AB, Basbaum AI (1998) Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 76(1-2):215–222
Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA (1994) Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res 37(5):596–603
Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J (2001) VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci 13(11):2105–2114
Matsuda M, Huh Y, Ji RR (2019) Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 33(1):131–139
Adelson D, Lao L, Zhang G, Kim W, Marvizón JC (2009) Substance P release and neurokinin 1 receptor activation in the rat spinal cord increase with the firing frequency of C-fibers. Neuroscience 161(2):538–553
Zhang H, Cang C-L, Kawasaki Y, Liang L-L, Zhang Y-Q, Ji R-R, Zhao Z-Q (2007) Neurokinin-1 receptor enhances trpv1 activity in primary sensory neurons via PKCε: a novel pathway for heat hyperalgesia. J Neurosci 27(44):12067–12077. https://doi.org/10.1523/jneurosci.0496-07.2007
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E (2019) The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol 33(2058738419838383):2058738419838383
Guo SH, Lin JP, Huang LE, Yang Y, Chen CQ, Li NN, Su MY, Zhao X, Zhu SM, Yao YX (2019) Silencing of spinal Trpv1 attenuates neuropathic pain in rats by inhibiting CAMKII expression and ERK2 phosphorylation. Sci Rep 9(1):019–39184
Teodoro FC, Tronco Júnior MF, Zampronio AR, Martini AC, Rae GA, Chichorro JG (2013) Peripheral substance P and neurokinin-1 receptors have a role in inflammatory and neuropathic orofacial pain models. Neuropeptides 47(3):199–206
Huang Z, Ma J, Chen J, Shen B, Pei F, Kraus VB (2015) The effectiveness of low-level laser therapy for nonspecific chronic low back pain: a systematic review and meta-analysis. Arthritis Res Ther 17(360):015–0882
de Andrade AL, Bossini PS, Parizotto NA (2016) Use of low level laser therapy to control neuropathic pain: a systematic review. J Photochem Photobiol B 164:36–42
de Oliveira MD, Martinez dos Santos F, Evany de Oliveira M, de Britto LR, Benedito Dias Lemos J, Chacur M (2013) Laser therapy and pain-related behavior after injury of the inferior alveolar nerve: possible involvement of neurotrophins. J Neurotrauma 30(6):480–486
Oliveira ME, Santos FM, Bonifácio RP, Freitas MF, Martins DO, Chacur M (2017) Low level laser therapy alters satellite glial cell expression and reverses nociceptive behavior in rats with neuropathic pain. Photochem Photobiol Sci 16(4):547–554
Martins DO, Marques DP, Venega RAG, Chacur M (2020) Photobiomodulation and B vitamins administration produces antinociception in an orofacial pain model through the modulation of glial cells and cytokines expression. Brain Behav Immun Health 2:100040. https://doi.org/10.1016/j.bbih.2020.100040
Pereira FC, Parisi JR, Maglioni CB, Machado GB, Barragán-Iglesias P, Silva JRT, Silva ML (2017) Antinociceptive effects of low-level laser therapy at 3 and 8 j/cm(2) in a rat model of postoperative pain: possible role of endogenous Opioids. Lasers Surg Med 49(9):844–851
Farivar S, Malekshahabi T, Shiari R (2014) Biological effects of low level laser therapy. J Lasers Med Sci 5(2):58–62
Cotler HB, Chow RT, Hamblin MR, Carroll J (2015) The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol 2(5):9
Graham ML, Prescott MJ (2015) The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur J Pharmacol 759:19–29
Giardini AC, Dos Santos FM, da Silva JT, de Oliveira ME, Martins DO, Chacur M (2017) Neural mobilization treatment decreases glial cells and brain-derived neurotrophic factor expression in the central nervous system in rats with neuropathic pain induced by cci in rats. Pain Res Manag 2017:7429761. https://doi.org/10.1155/2017/7429761
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1):87–107
Da Silva JT, Evangelista BG, Venega RAG, Oliveira ME, Chacur M (2017) Early and late behavioral changes in sciatic nerve injury may be modulated by nerve growth factor and substance P in rats: a chronic constriction injury long-term evaluation. J Biol Regul Homeost Agents 31(2):309–319
Balbinot LF, Robinson CC, Achaval M, Zaro MA, Brioschi ML (2013) Repeatability of infrared plantar thermography in diabetes patients: a pilot study. J Diabetes Sci Technol 7(5):1130–1137
Haddad DS, Brioschi ML, Baladi MG, Arita ES (2016) A new evaluation of heat distribution on facial skin surface by infrared thermography. Dentomaxillofac Radiol 45(4):19
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ellis A, Bennett DL (2013) Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111 (1):26-37
Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, Liu Q (2019) TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 10(1):019–13536
Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC (2007) The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 27(13):3366–3374
Gavva NR (2008) Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci 29(11):550–557
Sacharuk VZ, Lovatel GA, Ilha J, Marcuzzo S, Pinho AS, Xavier LL, Zaro MA, Achaval M (2011) Thermographic evaluation of hind paw skin temperature and functional recovery of locomotion after sciatic nerve crush in rats. Clinics 66(7):1259–1266
Bagavathiappan S, Philip J, Jayakumar T, Raj B, Rao PN, Varalakshmi M, Mohan V (2010) Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J Diabetes Sci Technol 4(6):1386–1392
Bostock H, Campero M, Serra J, Ochoa JL (2005) Temperature-dependent double spikes in C-nociceptors of neuropathic pain patients. Brain 128(9):2154–2163. https://doi.org/10.1093/brain/awh552
Pulst SM, Haller P (1981) Thermographic assessment of impaired sympathetic function in peripheral nerve injuries. J Neurol 226(1):35–42
Cline MA, Ochoa J, Torebjörk HE (1989) Chronic hyperalgesia and skin warming caused by sensitized C nociceptors. Brain 112(Pt 3):621–647
Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, Levy RM, Hunter CW (2019) A comprehensive algorithm for management of neuropathic pain. Pain Med 20(Suppl 1):S2–S12
Nesioonpour S, Mokmeli S, Vojdani S, Mohtadi A, Akhondzadeh R, Behaeen K, Moosavi S, Hojjati S (2014) The effect of low-level laser on postoperative pain after tibial fracture surgery: a double-blind controlled randomized clinical trial. Anesth Pain Med 4(3)
Acknowledgments
We thank A. Aldred for technical assistance.
Funding
This study was supported by Sao Paulo Research Foundation FAPESP (2013/01274-7, 2012/05840-4, 2010/20026-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
All procedures were approved by the Institutional Animal Care Committee of the University of São Paulo (protocol number 150/95—book number 02/2010).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mara Evany de Oliveira and Joyce Teixeira Da Silva share the first authorship.
Rights and permissions
About this article
Cite this article
de Oliveira, M.E., Da Silva, J.T., Brioschi, M.L. et al. Effects of photobiomodulation therapy on neuropathic pain in rats: evaluation of nociceptive mediators and infrared thermography. Lasers Med Sci 36, 1461–1467 (2021). https://doi.org/10.1007/s10103-020-03187-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03187-9