Abstract
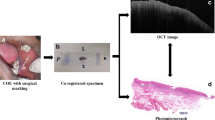
Surgery is still the first choice to treat oral cancer, where it is important to detect surgical margins in order to reduce cancer recurrence and maintain oral-maxillofacial function simultaneously. As a non-invasive and in situ imaging technique, optical coherence tomography (OCT) can obtain images close to the resolution of histopathology, which makes it have great potential in intraoperative diagnosis. However, it is not enough to find surgical margins accurately just observing OCT images directly and qualitatively. The purpose of this study is to identify oral cancer in OCT images by investigating the quantitative difference of cancer and non-cancer tissue. Based on an available optical attenuation model and the axial confocal PSF of a home-made swept source OCT system, by using fresh ex vivo human oral tissues from 14 patients of oral squamous cell carcinoma (OSCC) as the samples, diagnosis with sensitivity (97.88%) and specificity (83.77%) was achieved at the attenuation threshold of 4.7 mm−1, and the accuracy of identification reached 91.15% in our study. Our preliminary results demonstrated that the oral cancer resection will be guided accurately and the clinical application of OCT will be further promoted by deeply mining the information hidden in OCT images.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
American Cancer Society (2017) Cancer facts & figures. American Cancer Society, Atlanta
Massano J, Regateiro FS, Januario G, Ferreira A (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102(1):67–76. https://doi.org/10.1016/j.tripleo.2005.07.038
Azzopardi S, Lowe D, Rogers S (2019) Audit of the rates of re-excision for close or involved margins in the management of oral cancer. Br J Oral Maxillofac Surg 57(7):678–681. https://doi.org/10.1016/j.bjoms.2019.05.006
Costello F (2017) Optical coherence tomography in neuro-ophthalmology. Neurol Clin 35(1):153–163. https://doi.org/10.1016/j.ncl.2016.08.012
Abdelazeem K, Sharaf M, Saleh MGA, Fathalla AM, Soliman W (2018) Relevance of swept-source anterior segment optical coherence tomography for corneal imaging in patients with flap-related complications after LASIK. Cornea 38(1):93–97. https://doi.org/10.1097/ico.0000000000001773
Yonetsu T, Bouma BE, Kato K, Fujimoto JG, Jang IK (2013) Optical coherence tomography- 15 years in cardiology. Circ J 77(8):1933–1940. https://doi.org/10.1253/circj.cj-13-0643.1
Tsai TH, Leggett CL, Trindade AJ, Sethi A, Swager AF, Joshi V, Bergman JJ, Mashimo H, Nishioka NS, Namati E (2017) Optical coherence tomography in gastroenterology: a review and future outlook. J Biomed Opt 22(12):1–17. https://doi.org/10.1117/1.jbo.22.12.121716
Adabi S, Fotouhi A, Xu Q, Daveluy S, Mehregan D, Podoleanu A, Nasiriavanaki M (2018) An overview of methods to mitigate artifacts in optical coherence tomography imaging of the skin. Skin Res Technol 24(2):265–273. https://doi.org/10.1111/srt.12423
Jerjes W, Hamdoon Z, Hopper C (2019) Structural validation of facial skin using optical coherence tomography: a descriptive study. Skin Res Technol 00:1–10. https://doi.org/10.1111/srt.12791
Van Soest G, Goderie T, Regar E, Koljenovic S, van Leenders GLJH, Gonzalo N, van Noorden S, Okamura T, Bouma BE, Tearney GJ, Oosterhuis JW, Serruys PW, van der Steen AFW (2010) Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt 15(1):011105. https://doi.org/10.1117/1.3280271
Zhang Q, Wu X, Tang T, Zhu S, Yao Q, Gao B, Yuan X (2012) Quantitative analysis of rectal cancer by spectral domain optical coherence tomography. Phys Med Biol 57(16):5235–5244. https://doi.org/10.1088/0031-9155/57/16/5235
Xu C, Schmitt JM, Carlier SG, Virmani R (2008) Characterization of atherosclerosis plaques by measuring both backscattering and attenuation coefficients in optical coherence tomography. J Biomed Opt 13(3):034003
Kah JCY, Chow TH, Ng BK, Razul SG, Olivo M, Sheppard CJR (2009) Concentration dependence of gold nanoshells on the enhancement of optical coherence tomography images: a quantitative study. Appl Opt 48(10):D96–D108. https://doi.org/10.1364/AO.48.000D96
Yuan W, Kut C, Liang W, Li XD (2017) Robust and fast characterization of OCT-based optical attenuation using a novel frequency-domain algorithm for brain cancer detection. Sci Rep 7:44909. https://doi.org/10.1038/srep44909
Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, Rodriguez FJ, Quiñones-Hinojosa A, Li XD (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7(292):292ra100. https://doi.org/10.1126/scitranslmed.3010611
Tes D, Aber A, Zafar M, Horton L, Fotouhi A, Xu Q, Moiin A, Thompson AD, Moraes Pinto Blumetti TC, Daveluy S, Chen W, Nasiriavanaki M (2018) Granular cell tumor imaging using optical coherence tomography. Biomed Eng Comput Biol 9:1–9. https://doi.org/10.1177/1179597218790250
Adabi S, Hosseinzadeh M, Noei S, Conforto S, Daveluy S, Clayton A, Mehregan D, Nasiriavanaki M (2017) Universal in vivo textural model for human skin based on optical coherence tomograms. Sci Rep 7(1):17912. https://doi.org/10.1038/s41598-017-17398-8
Jung W, Zhang J, Chung J, Wilder-Smith P, Brenner M, Nelson JS, Chen Z (2005) Advances in oral cancer detection using optical coherence tomography. IEEE J Sel Top Quantum Electron 11(4):811–817. https://doi.org/10.1109/JSTQE.2005.857678
Wilder-Smith P, Hammer-Wilson MJ, Zhang J, Wang Q, Osann K, Chen Z, Wigdor H, Schwartz J, Epstein J (2007) In vivo imaging of oral mucositis in an animal model using optical coherence tomography and optical Doppler tomography. Clin Cancer Res 13(8):2449–2454. https://doi.org/10.1158/1078-0432.CCR-06-2234
Jerjes W, Upile T, Betz CS, Abbas S, Sandison A, Hopper C (2008) Detection of oral pathologies using optical coherence tomography. Eur Oncol Haematol 4(1):57–59. https://doi.org/10.17925/eoh.2008.04.1.57
Davoudi B, Lindenmaier A, Standish BA, Allo G, Bizheva K, Vitkin A (2012) Noninvasive in vivo structural and vascular imaging of human oral tissues with spectral domain optical coherence tomography. Biomed Opt Express 3(5):826–839. https://doi.org/10.1364/BOE.3.000826
Hamdoon Z, Jerjes W, Upile T, McKenzie G, Jay A, Hopper C (2013) Optical coherence tomography in the assessment of suspicious oral lesions: an immediate ex vivo study. Photodiagnosis and Photodynamic Therapy 10(1):17–27. https://doi.org/10.1016/j.pdpdt.2012.07.005
Choi WJ, Wang RK (2014) In vivo imaging of functional microvasculature within tissue beds of oral and nasal cavities by swept-source optical coherence tomography with a forward/side-viewing probe. Biomed Opt Express 5(8):2620–2634. https://doi.org/10.1364/BOE.5.002620
Maslennikova AV, Sirotkina MA, Moiseev AA, Finagina ES, Ksenofontov SY, Gelikonov GV, Matveev LA, Kiseleva EB, Zaitsev VY, Zagaynova EV, Feldchtein FI, Gladkova ND, Vitkin A (2017) In-vivo longitudinal imaging of microvascular changes in irradiated oral mucosa of radiotherapy cancer patients using optical coherence tomography. Sci Rep 7(1):16505. https://doi.org/10.1038/s41598-017-16823-2
Tsai M-T, Chen Y, Lee C-Y, Huang B-H, Trung NH, Lee Y-J, Wang Y-L (2017) Noninvasive structural and microvascular anatomy of oral mucosae using handheld optical coherence tomography. Biomed Opt Express 8(11):5001–5012. https://doi.org/10.1364/BOE.8.005001
Maslennikova A, Sirotkina M, Sedova E, Moiseev A, Ksenofontov S, Gelikonov G, Matveev L, Kiseleva E, Zaitsev V, Zagaynova E, Feldstein F, Gladkova N, Vitkin A (2018) In vivo imaging of microvascular changes in irradiated oral mucosa by optical coherence tomography. Radiother Oncol 127:E586–E586
Surlin P, Camen A, Stratul SI, Roman A, Gheorghe DN, Herascu E, Osiac E, Rogoveanu I (2018) Optical coherence tomography assessment of gingival epithelium inflammatory status in periodontal - systemic affected patients. Ann Anat 219:51–56. https://doi.org/10.1016/j.aanat.2018.04.010
Heidari AE, Sunny SP, James BL, Lam TM, Tran AV, Yu J, Ramanjinappa RD, Uma K, Birur P, Suresh A, Kuriakose MA, Chen Z, Wilder-Smith P (2019) Optical coherence tomography as an oral cancer screening adjunct in a low resource settings. IEEE J Sel Top Quantum Electron 25(1):1–8. https://doi.org/10.1109/JSTQE.2018.2869643
Tsai M-T, Lee H-C, Lee C-K, Yu C-H, Chen H-M, Chiang C-P, Chang C-C, Wang Y-M, Yang CC (2008) Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt Express 16(20):15847–15862. https://doi.org/10.1364/OE.16.015847
Tsai M-T, Lee C-K, Lee H-C, Chen H-M, Chiang C-P, Wang Y-M, Yang CC (2009) Differentiating oral lesions in different carcinogenesis stages with optical coherence tomography. J Biomed Opt 14(4):044028. https://doi.org/10.1117/1.3200936
Adegun OK, Tomlins PH, Hagi-Pavli E, McKenzie G, Piper K, Bader DL, Fortune F (2012) Quantitative analysis of optical coherence tomography and histopathology images of normal and dysplastic oral mucosal tissues. Lasers Med Sci 27(4):795–804. https://doi.org/10.1007/s10103-011-0975-1
Faber DJ, van der Meer FJ, Aalders MCG, van Leeuwen TG (2004) Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Opt Express 12(19):4353–4365. https://doi.org/10.1364/OPEX.12.004353
Adegun OK, Tomlins PH, Hagi-Pavli E, Bader DL, Fortune F (2012) Quantitative optical coherence tomography of fluid-filled oral mucosal lesions. Lasers Med Sci 28(5):1249–1255. https://doi.org/10.1007/s10103-012-1208-y
Fatakdawala H, Poti S, Zhou F, Sun Y, Bec J, Liu J, Yankelevich DR, Tinling SP, Gandour-Edwards RF, Farwell DG, Marcu L (2013) Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques. Biomed Opt Express 4(9):1724–1741. https://doi.org/10.1364/BOE.4.001724
Izzetti R, Fantoni G, Gelli F, Faggioni L, Vitali S, Gabriele M, Caramella D (2018) Feasibility of intraoral ultrasonography in the diagnosis of oral soft tissue lesions: a preclinical assessment on an ex vivo specimen. Radiol Med 123(2):135–142. https://doi.org/10.1007/s11547-017-0817-8
Simonato LE, Tomo S, Miyahara GI, Navarro RS, Villaverde AGJB (2017) Fluorescence visualization efficacy for detecting oral lesions more prone to be dysplastic and potentially malignant disorders: a pilot study. Photodiagn Photodyn Ther 17:1–4. https://doi.org/10.1016/j.pdpdt.2016.10.010
Lu G, Wang D, Qin X, Muller S, Wang X, Chen AY, Chen ZG, Fei B (2018) Detection and delineation of squamous neoplasia with hyperspectral imaging in a mouse model of tongue carcinogenesis. J Biophotonics 11(3). https://doi.org/10.1002/jbio.201700078
Wang J, Zheng W, Lin K, Huang Z (2018) Characterizing biochemical and morphological variations of clinically relevant anatomical locations of oral tissue in vivo with hybrid Raman spectroscopy and optical coherence tomography technique. J Biophotonics 11(3). https://doi.org/10.1002/jbio.201700113
Tsai M-R, Shieh D-B, Lou P-J, Lin C-F, Sun C-K (2012) Characterization of oral squamous cell carcinoma based on higher-harmonic generation microscopy. J Biophotonics 5(5–6):415–424. https://doi.org/10.1002/jbio.201100144
Turani Z, Fatemizadeh E, Blumetti T, Daveluy S, Moraes AF, Chen W, Mehregan D, Andersen PE, Nasiriavanaki M (2019) Optical radiomic signatures derived from optical coherence tomography images to improve identification of melanoma. Cancer Res 79(8):2021–2030
Yang Z, Shang J, Liu C, Zhang J, Hou F, Liang Y (2020) Intraoperative imaging of oral-maxillofacial lesions using optical coherence tomography. J Innov Opt Health Sci:2050010. https://doi.org/10.1142/s1793545820500108
Funding
This study was supported in part by the National Natural Science Foundation of China (61875092 and 11374167), Science and Technology Support Program of Tianjin (17YFZCSY00740), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of Tianjin Stomatological Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Shang, J., Liu, C. et al. Identification of oral cancer in OCT images based on an optical attenuation model. Lasers Med Sci 35, 1999–2007 (2020). https://doi.org/10.1007/s10103-020-03025-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03025-y