Abstract
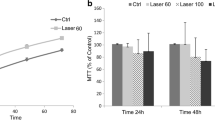
The aim of this study was to evaluate the influence of low-level laser therapy (LLLT) with different parameters and wavelengths on nitric oxide (NO) release and cell viability. Irradiation was performed with Ga-Al-As laser, continuous mode and wavelengths of 660 and 808 nm at different energy and power densities. For each wavelength, powers of 30, 50, and 100 mW and times of 10, 30, and 60 s were used. NO release was measured using Griess reaction, and cell viability was evaluated by mitochondrial reduction of bromide 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to formazan. LLLT promoted statistically significant changes in NO release and MTT value only at the wavelength of 660 nm (p < 0.05). LLLT also promoted an increase in the NO release and cell viability when the energy densities 64 (p = 0.04) and 214 J/cm2 (p = 0.012), respectively, were used. LLLT has a significant impact on NO release without affecting cell viability, but the significance of these findings in the inflammatory response needs to be further studied.


Similar content being viewed by others
References
Moriyama Y, Nguyen J, Akens M et al (2009) In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med 41:227–231
Pinheiro SL, Donegá LM, Seabra LMS et al (2010) Capacity of photodynamic therapy for microbial reduction in periodontal pockets. Lasers Med Sci 25:87–91
Frozanfar A, Ramezani M, Rahpeyma A et al (2013) The effects of low level laser therapy on the expression of collagen type I gene and proliferation of human gingival fibroblasts (Hgf3-Pi 53): in vitro study. Iran J Basic Med Sci 16:1071–1074
Almeida Lopes L, Rigau J, Zangaro RA et al (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Kreisler M, Christoffers AB, Al-Haj H et al (2002) Low level 809 nm diode laser induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers Surg Med 30:365–392
Rocha AM Jr, Andrade LCF, Oliveira RG et al (2006) Modulação da proliferação fibroblástica e da resposta inflamatória pela terapia a laser de baixa intensidade no processo de reparo tecidual. An Bras Dermatol 81:150–156
Ribeiro MS, Da Silva Dde F, De Araujo CE et al (2004) Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study. J Clin Laser Med Surg 22:59–66
Hagiwara S, Iwasaka H, Hasegawa A et al (2010) Pre-irradiation of blood by gallium aluminum arsenide (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Anesth Analg 107:1058–1063
Mizutani K, Musya Y, Wakae K et al (2008) A clinical study on serum prostaglandin E2 with low-level laser therapy. Photomed Laser Surg 22:537–539
Meneguzzo DT, Lopes LA, Pallota R et al (2013) Prevention and treatment of mice paw edema by near-infrared low-level laser therapy on lymph nodes. Lasers Med Sci 28:973–980
Albertini R, Villa Verde AB, Aimbire F et al (2008) Cytokines mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low level laser therapy (LLLT). Photomed Laser Surg 26:19–24
Lima FM, Villaverde AB, Albertini R et al (2011) Dual effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg Med 43:410–420
Boschi ES, Leite CE, Saciura VC et al (2008) Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med 40:500–508
Lima FM, Vitoretti L, Coelho F et al (2013) Suppressive effect of low-level laser therapy on tracheal hyperresponsiveness and lung inflammation in rat subjected to intestinal ischemia and reperfusion. Lasers Med Sci 28:551–564
Safavi SM, Kazemi B, Esmaeili M et al (2008) Effects of low-level He–Ne laser irradiation on the gene expression of IL-1b, TNF-a, IFNc, TGF-b, bFGF, and PDGF in rat’s gingival. Lasers Med Sci 3:331–335
Lowenstein CJ, Padalko E (2014) iNOS (NOS2) at a glance. J Cell Sci 117:2865–2867
Nathan C (2002) Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Servetto N, Cremonezzi D, Simes JC et al (2010) Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers Surg Med 42:577–583
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916
Lancaster JR (1992) Nitric oxide in cells. Am Sci 80:248–259
Tunér J, Hode L (2002) Laser therapy: clinical practice and scientific background. Prima Books, Sweden
Moriyama Y, Moriyarna EH, Blackmore K, Akens MK, Lilge L (2005) In vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imaging. Photochem Photobiol 8:1351–1355
Gavish L, Perez LS, Reissman P (2008) Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg Med 40:371–378
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245
Huang TH, Lu YC, Kao CT (2012) Low-level diode laser therapy reduces lipopolysaccharide (LPS)-induced bone cell inflammation. Lasers Med Sci 27:621–627
Silva Garcez A, Simões Ribeiro M, Núñez SC (2012) Laser de baixa potência: princípios básicos e aplicações clínicas na Odontologia. Elsevier, Rio de Janeiro
Novoselova EG, Glushkova OV, Cherenkov DA et al (2006) Effects of low-power laser radiation on mice immunity. Photodermatol Photoimmunol Photomed 22:33–38
Huang Y-Y, Chen ACH, Carrol JD et al (2009) Biphasic dose response in low level light therapy. Dose-Response 7:358–383
Albertini R, Aimbire FSC, Correa FI et al (2004) Effects of different protocol doses of low power gallium-aluminum-arsenate (Ga-Al-As) laser radiation (650 nm) on carrageenan induced rat paw oedema. J Photochem Photobiol 74:101–107
Karu T (2003) Low power laser therapy. In: Biomedics photonics handbook. CRC Press, Tennessee, Boca Raton
Kroncke KD, Fehsel K, Kolb-Bachofen V (1997) Nitric oxide: cytotoxicity versus cytoprotection—how, why, when, and where? Nitric Oxide 1:107–120
Wilden L, Karthein R (1998) Import of phenomena of electrons and therapeutic low-level laser in regard to the mitochondrial energy transfer. J Clin Laser Med Surg 16:159–165
Wahl SM, McCartney-Francis N, Chan R, Dionne J et al (2003) Nitric oxide in experimental joint inflammation. Benefit or detriment? Cells Tissues Organs 174:26–33
van Breugel HH, Bär PR (1992) Power and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528–537
Azevedo LH, de Paula Eduardo F, Moreira MS et al (2006) Influence of different power densites of LILT on cultured human fibroblast growth: a pilot study. Laser Med Sci 21:86–89
Raetz CR, Ulevitch RJ, Wright SD et al (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5:2652–2660
Wu CH, Chen TL, Chen TG et al (2003) Nitric oxide modulates pro- and anti-inflammatory cytokines in lipopolysaccharide-activated macrophages. J Trauma 55:540–545
Sies H (2007) Biological redox systems and oxidative stress. Cell Mol Life Sci 64:2181–2188
Dávila S, Vignola MB, Cremonezzi D et al (2011) Low level laser therapy on experimental myopathy. Laser Ther 20:287–292
Aimbire F, Albertini R, Pacheco MT et al (2006) Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg 24:33–37
Vladimirov, Y.A, A. N.; Klebanov, G. I. (2004) Photobiological principles of therapeutic applications of laser radiation. Review. Biochemistry. 69:81–90.
Gavish L, Perez L, Gertz SD (2006) Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Lasers Surg Med 38:779–786
Schmidt A, Geigenmueller S, Voelker W et al (2003) Exogenous nitric oxide causes overexpression of TGFbeta1 and overproduction of extracellular matrix in human coronary smooth muscle cells. Cardiovasc Res 58:671–678
Kuwabara M, Kakinuma Y, Ando M et al (2006) Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J Physiol Sci 56:95–101
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Silva, I.H.M., de Andrade, S.C., de Faria, A.B.S. et al. Increase in the nitric oxide release without changes in cell viability of macrophages after laser therapy with 660 and 808 nm lasers. Lasers Med Sci 31, 1855–1862 (2016). https://doi.org/10.1007/s10103-016-2061-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2061-1