Abstract
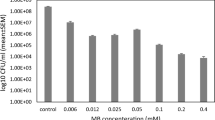
The treatment of Klebsiella pneumoniae, particularly extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae, is currently a great challenge. Photodynamic antimicrobial chemotherapy is a promising approach for killing antibiotic-resistant bacteria. The aim of this study was to evaluate the capacity of 5-aminolevulinic acid (5-ALA) and its derivative 5-ALA methyl ester (MAL) in the presence of white light to cause photodynamic inactivation (PDI) of K. pneumoniae planktonic and biofilm cells. In the presence of white light, 5-ALA and MAL inactivated planktonic cells in a concentration-dependent manner. Biofilms were also sensitive to 5-ALA and MAL-mediated PDI. The mechanisms by which 5-ALA and MAL caused PDI of ESBL-producing K. pneumonia were also investigated. Exposure of K. pneumonia to light in the presence of either 5-ALA or MAL induced cleavage of genomic DNA and the rapid release of intracellular biopolymers. Intensely denatured cytoplasmic contents and aggregated ribosomes were also detected by transmission electron microscopy. Scanning electron microscopy showed that PDI of biofilms caused aggregated bacteria to detach and that the bacterial cell envelope was damaged. This study provides insights into 5-ALA and MAL-mediated PDI of ESBL-producing K. pneumoniae.






Similar content being viewed by others
References
Shon AS, Bajwa RPS, Russo TA (2013) Hypervirulent Klebsiella pneumoniae: a new and dangerous breed. Virulence 4(2):107–118
Pitout JDD, Laupland KB (2008) Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8(3):159–166
Bradford PA (2001) Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14(4):933–951
Vardakas KZ et al (2012) Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. doi:10.1093/jac/dks301
Denis TGS et al (2011) All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2(6):509–520
Celli JP et al (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 110(5):2795–2838
Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumor immunity. Nat Rev Cancer 6:535–545
Wood S et al (2006) Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 57(4):680–684
Komerik N, Wilson M (2002) Factors influencing the susceptibility of gram-negative bacteria to toluidine blue O-mediated lethal photosensitization. J Appl Microbiol 92(4):618–623
Haidaris CG et al (2013) Effective photodynamic therapy against microbial populations in human deep tissue abscess aspirates. Laser Surg Med 45(8):509–516
Rossoni RD et al (2010) Comparison of the efficacy of rose bengal and erythrosine in photodynamic therapy against Enterobacteriaceae. Laser Med Sci 25(4):581–596
Harris F, Pierpoint L (2012) Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Med Res Rev 32(6):1292–1327
Li X et al (2013) Effects of 5-aminolevulinic acid-mediated photodynamic therapy on antibiotic-resistant staphylococcal biofilm: an in vitro study. J Surg Res 184(2):1013–1021
Fotinos N et al (2008) Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic acid and 5-aminolevulinic acid derivatives. Animicrob Agents Chemother 52(4):1366–1373
Nitzan Y et al (2004) ALA induced photodynamic effects on gram positive and negative bacteria. Photochem Photobiol Sci 3:430–435
Yow CMN, Fung K, Wong KC (2011) Photodynamic inactivation of multi-drug resistant pathogens in Hong Kong. Hong Kong Med J 17(Suppl 2):S24–28
Peng Z et al (2011) Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob Agents Chemother 55(2):860–866
Chen CZ, Cooper SL (2002) Interactions between dendrimer biocides and bacterial membranes. Biomaterials 23(16):3359–3368
Je JY, Kim SK (2006) Antimicrobial action of novel chitin derivative. Biochim Biophys Acta 1760(1):104–109
Spesia MB et al (2009) Mechanistic insight of the photodynamic inactivation of Escherichia coli by a tetracationic zinc(II) phthalocyanine derivative. Photodiagn Photodyn Ther 6(1):52–61
Spesia MB, Duraniti EN (2013) Photodynamic inactivation mechanism of Streptococcus mitis sensitized by zinc (II) 2, 9, 16, 23-tetrakis [2-(N, N, N-trimethylamino) ethoxy] phthalocyanine. J Photochem Photobiol B 125(5):179–187
Caminos DA et al (2008) Mechanisms of Escherichia coli photodynamic inactivation by an amphiphilic tricationic porphyrin and 5, 10, 15, 20-tetra (4-N, N, N-trimethylammoniumphenyl) porphyrin. Photochem Photobiol Sci 7:1071–1078
Fotinos N et al (2006) 5-Aminolevulinic acid derivatives in photomedicine: characteristics, application and perspectives. Photochem Photobiol 82(4):994–1015
Demidova T, Hamblin M (2005) Effects of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother 49(6):2329–2335
Sutherland IW (2001) The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol 9(5):222–227
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(9276):135–138
Lee C et al (2004) 5-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J Photochem Photobiol B 75(1–2):21–25
Mah TFC, Toole GAO’ (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9(1):34–39
Gad F et al (2004) Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother 48(6):2173–2178
Bertoloni G et al (2000) Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochim Biophys Acta 1475(2):169–174
Capella M, Coelho AM, Menezes S (1996) Effect of glucose on photodynamic action of methylene blue in Escherichia coli cells. Photochem Photobiol 64(1):205–210
Choi SS, Lee HK, Chae HS (2012) Comparison of in vitro photodynamic antimicrobial activity of protoporphyrin IX between endoscopic white light and newly developed narrowband endoscopic light against Helicobacter pylori 26695. J Photochem Photobiol B 117(5):55–60
Nitzan Y, Ashkenazi H (2001) Photoinactivation of Acinetobacter baumannii and Escherichia coli B by cationic hydrophilic porphyrin at various light wavelengths. Curr Microbiol 42(6):408–414
Acknowledgments
We are really grateful for assistance from Professor Kewu Yang at the College of Chemistry and Materials Science, Northwest University, China. This work was supported by the National Natural Science Foundation of China (81401710), China Postdoctoral Science Foundation (2014M562424 and 2015T81034), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liu, C., Zhou, Y., Wang, L. et al. Photodynamic inactivation of Klebsiella pneumoniae biofilms and planktonic cells by 5-aminolevulinic acid and 5-aminolevulinic acid methyl ester. Lasers Med Sci 31, 557–565 (2016). https://doi.org/10.1007/s10103-016-1891-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1891-1